Journal of Allergy and Clinical Immunology ( IF 14.2 ) Pub Date : 2020-10-23 , DOI: 10.1016/j.jaci.2020.09.039 Mark Rochman 1 , Yong Mei Xie 1 , Lydia Mack 1 , Julie M Caldwell 1 , Andrea M Klingler 1 , Garrett A Osswald 1 , Nurit P Azouz 2 , Marc E Rothenberg 2
|
Background
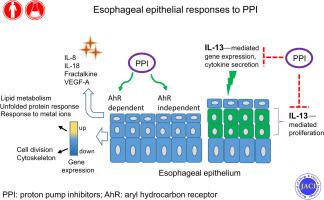
Proton pump inhibitors (PPIs) have been recognized as a primary treatment of eosinophilic esophagitis (EoE), an allergic inflammatory disease of the esophageal mucosa. The mechanisms underlying esophageal epithelial responses to PPIs remain poorly understood.
Objective
We hypothesized that PPIs can counteract IL-13–mediated esophageal epithelial responses that are germane for EoE pathogenesis.
Methods
Transcriptional responses of human esophageal cells to IL-13 and the PPIs omeprazole and esomeprazole were assessed by RT-PCR and RNA sequencing. Cytokine secretion was measured by multiplex analysis and ELISA.
Results
Human esophageal epithelial cells robustly responded to PPI stimulation by inducing a set of 479 core genes common between omeprazole and esomeprazole treatments. The transcriptional response to PPIs was partially mediated through the aryl hydrocarbon receptor signaling pathway, as the aryl hydrocarbon receptor antagonist GNF-351 modified approximately 200 genes, particularly those enriched in metabolic processes and regulation of cell death. PPI treatment reversed approximately 20% of the IL-13 transcriptome. Functional analysis of the PPI-responsive, upregulated genes revealed enrichment in metabolic and oxidation processes, and the unfolded protein response. In contrast, downregulated genes were overrepresented in functional terms related to cell division and cytoskeletal organization, which were also enriched for the genes in the EoE transcriptome reversed by PPIs. Furthermore, PPI treatment decreased the IL-13–induced proliferative response of esophageal epithelial cells.
Conclusions
These results demonstrate broad effects of PPIs on esophageal epithelium, including their ability to curtail transcriptomic processes involved in cellular proliferation and IL-13–induced responses, and they highlight the importance of AHR signaling in mediating these responses.
中文翻译:

人食管上皮对质子泵抑制剂的广泛转录反应
背景
质子泵抑制剂 (PPI) 已被公认为嗜酸性食管炎 (EoE) 的主要治疗方法,EoE 是一种食管黏膜的过敏性炎症性疾病。食管上皮对 PPI 反应的机制仍然知之甚少。
客观的
我们假设 PPI 可以抵消与 EoE 发病机制密切相关的 IL-13 介导的食管上皮反应。
方法
通过 RT-PCR 和 RNA 测序评估人食管细胞对 IL-13 和 PPI 奥美拉唑和埃索美拉唑的转录反应。通过多重分析和ELISA测量细胞因子分泌。
结果
人类食管上皮细胞通过诱导奥美拉唑和埃索美拉唑治疗之间共有的一组 479 个核心基因对 PPI 刺激作出强烈反应。对 PPI 的转录反应部分是通过芳烃受体信号通路介导的,因为芳烃受体拮抗剂 GNF-351 修饰了大约 200 个基因,特别是那些富含代谢过程和细胞死亡调节的基因。PPI 处理逆转了大约 20% 的 IL-13 转录组。对 PPI 反应性上调基因的功能分析揭示了代谢和氧化过程的富集,以及未折叠的蛋白质反应。相比之下,下调的基因在与细胞分裂和细胞骨架组织相关的功能方面被过度表达,它们还富含被 PPI 逆转的 EoE 转录组中的基因。此外,PPI 治疗降低了 IL-13 诱导的食管上皮细胞增殖反应。
结论
这些结果证明了 PPI 对食管上皮的广泛影响,包括它们减少参与细胞增殖和 IL-13 诱导反应的转录组过程的能力,并且它们强调了 AHR 信号传导在介导这些反应中的重要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号