Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-10-21 , DOI: 10.1016/j.bmcl.2020.127612 Srishylam Penjarla 1 , Subir Kumar Sabui 2 , Dhande Sudhakar Reddy 2 , Shyamapada Banerjee 2 , Paidi Yella Reddy 2 , Santhosh Penta 3 , Yogesh S Sanghvi 4
|
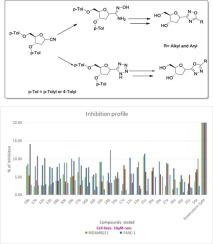
Various tetrazole and oxadiazole C-nucleoside analogues were synthesized starting from pure α- or β-glycosyl-cyanide. The synthesis of glycosyl-cyanide as key precursor was optimized on gram-scale to furnish crystalline starting material for the assembly of C-nucleosides. Oxadizole C-nucleosides were synthesized via two independent routes. First, the glycosyl-cyanide was converted into an amidoxime which upon ring closure offered an alternative pathway for the assembly of 1,2,4-oxadizoles in an efficient manner. Second, both anomers of glycosyl-cyanide were transformed into tetrazole nucleosides followed by acylative rearrangement to furnish 1,3,4-oxadiazoles in high yields. These protocols offer an easy access to otherwise difficult to synthesize C-nucleosides in good yield and protecting group compatibility. These C-nucleosides were evaluated for their antitumor activity. This work paves a path for facile assembly of library of new chemical entities useful for drug discovery.
中文翻译:

有效合成新型2'-脱氧-C-核苷的四唑和恶二唑类似物及其抗肿瘤活性
从纯的α-或β-糖基氰化物开始合成了各种四唑和恶二唑C-核苷类似物。在克规模上优化了作为主要前体的糖基氰化物的合成,以提供用于C-核苷组装的结晶原料。草酸二唑C-核苷是通过两种独立的途径合成的。首先,将糖基氰化物转化为an胺肟,其在闭环时为有效地组装1,2,4-乙二唑提供了另一种途径。其次,将糖基氰化物的两个端基异构体都转化为四唑核苷,然后进行酰基化重排,从而以高收率提供了1,3,4-恶二唑。这些协议可轻松获得原本难以合成的C-核苷,并具有良好的收率和保护基团的兼容性。评价这些C-核苷的抗肿瘤活性。这项工作为便利用于药物发现的新化学实体库的组装铺平了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号