Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2020-10-23 , DOI: 10.1016/j.bbagen.2020.129773 Jose Kaneti , Milena Georgieva , Miroslav Rangelov , Irena Philipova , Bela Vasileva , Ivan Angelov , Dessislava Staneva , George Miloshev , Snezhana Bakalova
|
Background
Quinazolines 1 to 6, with an aromatic or aryl-vinyl substituent in position 2 are selected with the aim to compare their structures and biological activity. The selection includes a natural alkaloid, schizocommunin, and the synthetic 2-(2′-quinolyl)-3H-quinazolin-4-one, known to interact with guanine-quadruplex dependent enzymes, respectively telomerase and topoisomerase.
Methods
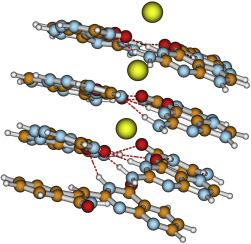
Breast cancer cells of the MDA cell line have been used to study the bioactivity of the tested compounds by the method of Comet Assay and FACS analyses. We model observed effects assuming stacking interactions of studied heterocycles with a naked skeleton of G-quadruplex, consisting of guanine quartet layers and potassium ions. Interaction energies are computed using a dispersion corrected density functional theory method, and an electron-correlated molecular orbital theory method.
Results
Selected compounds do not remarkably delay nor change the dynamics of cellular progression through the cell cycle phases, while changing significantly cell morphology. Our computational models quantify structural effects on heterocyclic G4-complex stabilization energies, which directly correlate with observed biological activity.
Conclusion
Our computational model of G-quadruplexes is an acceptable tool for the study of interaction energies of G-quadruplexes and heterocyclic ligands, predicting, and allowing design of novel structures.
General significance
Genotoxicity of quinazolin-4-one analogues on human breast cancer cells is not related to molecular metabolism but rather to their interference with G-quadruplex regulatory mechanisms. Computed stabilization energies of heterocyclic ligand complexes of G-quadruplexes might be useful in the prediction of novel telomerase / helicase, topoisomerase and NA polymerase dependent drugs.
中文翻译:

喹唑啉类似物的生物活性及其与G-四链体相互作用的分子模型
背景
选择在位置2具有芳族或芳基-乙烯基取代基的喹唑啉1至6,以比较它们的结构和生物学活性。Ť ħ Ë选择包括天然生物碱,schizocommunin,合成2-(2'-喹啉基)-3H-喹唑啉-4-酮,已知的相互作用与鸟嘌呤-四联依赖性酶,分别端粒酶和拓扑异构酶。
方法
通过彗星试验和FACS分析的方法,已经使用MDA细胞系的乳腺癌细胞来研究被测化合物的生物活性。我们对观察到的效应进行建模,假设研究的杂环与G-四链体的裸露骨架(由鸟嘌呤四重奏层和钾离子组成)的堆叠相互作用。使用色散校正的密度泛函理论方法和电子相关分子轨道理论方法计算相互作用能。
结果
所选择的化合物在显着改变细胞形态的同时,不会显着延迟或改变整个细胞周期阶段的细胞进程动态。我们的计算模型量化了对杂环G4络合物稳定能的结构影响,这与观察到的生物活性直接相关。
结论
我们的G-四链体计算模型是研究G-四链体与杂环配体相互作用能,预测并允许设计新结构的可接受工具。
一般意义
喹唑啉-4-酮类似物对人乳腺癌细胞的遗传毒性与分子代谢无关,而与它们干扰G-四链体调控机制有关。G-四链体的杂环配体复合物的计算稳定能可能对预测新型端粒酶/解旋酶,拓扑异构酶和NA聚合酶依赖性药物有用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号