Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-10-29 , DOI: 10.1016/j.bbamem.2020.183497 Maryssa Beasley 1 , Sharon Groover 1 , Stephen J Valentine 1 , Justin Legleiter 2
|
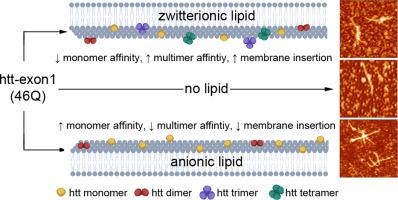
Huntington's Disease is a fatal neurodegenerative disorder caused by expansion of a glutamine repeat region (polyQ) beyond a critical threshold within exon1 of the huntingtin protein (htt). As a consequence of polyQ expansion, htt associates into a variety of aggregate species that are thought to underlie cellular toxicity. Within cells, htt associates with numerous membranous organelles and surfaces that exert influence on the aggregation process. In particular, the first 17 amino acids at the N-terminus of htt (Nt17) serve as a lipid-binding domain that is intrinsically disordered in bulk solution but adopts an amphipathic α-helical structure upon binding membranes. Beyond this, Nt17 is implicated in initiating htt fibrillization. As the interaction between Nt17 and lipid membranes is likely influenced by lipid properties, the impact of lipid headgroups on htt-exon1 aggregation, membrane activity, and the ability to form protein:lipid complexes was determined. Htt-exon1 with a disease-length polyQ domain (46Q) was exposed to lipid vesicles comprised of lipids with either zwitterionic (POPC and POPE) or anionic (POPG and POPS) headgroups. With zwitterionic head groups, large lipid to peptide ratios were required to have a statistically significant impact on htt aggregation. Anionic lipids enhanced htt fibrillization, even at low lipid:protein ratios, and this was accompanied by changes in aggregate morphology. Despite the larger impact of anionic lipids, htt-exon1(46Q) was more membrane active with zwitterionic lipid systems. The ability of Nt17 to form complexes with lipids was also mediated by lipid headgroups as zwitterionic ionic lipids more readily associated with multimeric forms of Nt17 in comparison with anionic lipids. Collectively, these results highlight the complexity of htt/membrane interactions and the resulting impact on the aggregation process.
中文翻译:

脂质头基改变膜上亨廷顿蛋白的聚集
亨廷顿氏病是一种致命的神经退行性疾病,由谷氨酰胺重复区域 (polyQ) 的扩张超出亨廷顿蛋白 (htt) 外显子 1 的临界阈值引起。作为 polyQ 扩展的结果,htt 与各种被认为是细胞毒性基础的聚合物种相关联。在细胞内,htt 与许多对聚集过程产生影响的膜细胞器和表面相关联。特别是,htt N 末端的前 17 个氨基酸 (Nt17) 作为脂质结合域,在散装溶液中本质上是无序的,但在结合膜时采用两亲性 α-螺旋结构。除此之外,Nt17 与启动 htt 纤维化有关。由于 Nt17 和脂质膜之间的相互作用可能受脂质特性的影响,确定了脂质头基对 htt-exon1 聚集、膜活性和形成蛋白质:脂质复合物的能力的影响。具有疾病长度 polyQ 结构域 (46Q) 的 Htt-exon1 暴露于由具有两性离子(POPC 和 POPE)或阴离子(POPG 和 POPS)头部基团的脂质组成的脂质囊泡。对于两性离子头部基团,需要大的脂质与肽的比例才能对 htt 聚集产生统计学上的显着影响。阴离子脂质增强 htt 纤维化,即使在低脂质:蛋白质比率下,这也伴随着聚集形态的变化。尽管阴离子脂质的影响更大,但 htt-exon1(46Q) 在两性离子脂质系统中更具膜活性。Nt17 与脂质形成复合物的能力也由脂质头基介导,因为与阴离子脂质相比,两性离子离子脂质更容易与 Nt17 的多聚体形式相关联。总的来说,这些结果突出了 htt/膜相互作用的复杂性以及由此产生的对聚集过程的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号