当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The molecular bases of chiral recognition in 2-(benzylsulfinyl)benzamide enantioseparation
Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.aca.2020.10.050 Paola Peluso , Bezhan Chankvetadze
Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.aca.2020.10.050 Paola Peluso , Bezhan Chankvetadze
|
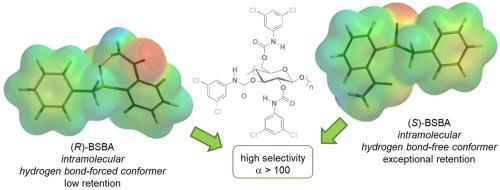
Liquid-phase chromatography on chiral stationary phase is still the most popular and versatile technique to separate enantiomers, which is based on the ability of a chiral selector (CS) to recognize the enantiomers of a chiral compound in a solvating medium. The knowledge of the molecular bases of the enantiodiscrimination process is a basic requirement to approach rationally the enantioseparation task. Indeed, analyte, CS, and mobile phase (MP) being the pivotal components of the chromatographic system, their properties, functions and mutual noncovalent interactions determine the enantioseparation outcome. In the last few decades, focused computational methods and techniques have been integrating experimental data and applying for the comprehension of the enantiorecognition phenomenon at molecular level. In this context, for understanding of molecular mechanisms of chiral recognition in separation of enantiomers, we propose a computational procedure based on conformational and electrostatic potential (V) analysis of both analyte and selector. First, low-energy conformers of the analyte were identified by conformational search, which occurring potentially on the selector surface. Then, local electron charge density of specific molecular regions of the interacting partners were inspected in terms of calculated V. This approach was used to explore at molecular level the enantioseparation mechanism of 2-(benzylsulfinyl)benzamide on cellulose-based CSs. By correlating calculated properties with experimental chromatographic parameters available in the literature, the structural landscape of the analyte and CSs in the enantiodiscrimination event and the differences between potential competing sites were profiled. A conformational transition of analyte structure on the CS surface was found to originate the exceptional enantioseparation of the 2-(benzylsulfinyl)benzamide (α > 100). Importantly, the proposed computational analysis provides a rationale of why and how the analytical separation occurs.
中文翻译:

2-(苄基亚磺酰基)苯甲酰胺对映分离中手性识别的分子基础
手性固定相上的液相色谱仍然是分离对映体的最流行和通用的技术,它基于手性选择器 (CS) 识别溶剂化介质中手性化合物对映体的能力。对映体区分过程的分子基础知识是合理处理对映体分离任务的基本要求。事实上,分析物、CS 和流动相 (MP) 是色谱系统的关键组成部分,它们的特性、功能和相互的非共价相互作用决定了对映分离结果。在过去的几十年里,重点计算方法和技术一直在整合实验数据并应用于分子水平的对映识别现象的理解。在这种情况下,为了理解对映异构体分离中手性识别的分子机制,我们提出了一种基于分析物和选择器的构象和静电势 (V) 分析的计算程序。首先,分析物的低能量构象异构体通过构象搜索来识别,这可能发生在选择器表面。然后,根据计算的 V 检查相互作用伙伴特定分子区域的局部电子电荷密度。该方法用于在分子水平上探索 2-(苄基亚磺酰基)苯甲酰胺在基于纤维素的 CS 上的对映分离机制。通过将计算的特性与文献中可用的实验色谱参数相关联,对映体区分事件中分析物和 CS 的结构景观以及潜在竞争位点之间的差异进行了分析。发现 CS 表面上分析物结构的构象转变起源于 2-(苄基亚磺酰基) 苯甲酰胺 (α > 100) 的特殊对映分离。重要的是,提议的计算分析提供了分析分离发生的原因和方式的基本原理。
更新日期:2021-01-01
中文翻译:

2-(苄基亚磺酰基)苯甲酰胺对映分离中手性识别的分子基础
手性固定相上的液相色谱仍然是分离对映体的最流行和通用的技术,它基于手性选择器 (CS) 识别溶剂化介质中手性化合物对映体的能力。对映体区分过程的分子基础知识是合理处理对映体分离任务的基本要求。事实上,分析物、CS 和流动相 (MP) 是色谱系统的关键组成部分,它们的特性、功能和相互的非共价相互作用决定了对映分离结果。在过去的几十年里,重点计算方法和技术一直在整合实验数据并应用于分子水平的对映识别现象的理解。在这种情况下,为了理解对映异构体分离中手性识别的分子机制,我们提出了一种基于分析物和选择器的构象和静电势 (V) 分析的计算程序。首先,分析物的低能量构象异构体通过构象搜索来识别,这可能发生在选择器表面。然后,根据计算的 V 检查相互作用伙伴特定分子区域的局部电子电荷密度。该方法用于在分子水平上探索 2-(苄基亚磺酰基)苯甲酰胺在基于纤维素的 CS 上的对映分离机制。通过将计算的特性与文献中可用的实验色谱参数相关联,对映体区分事件中分析物和 CS 的结构景观以及潜在竞争位点之间的差异进行了分析。发现 CS 表面上分析物结构的构象转变起源于 2-(苄基亚磺酰基) 苯甲酰胺 (α > 100) 的特殊对映分离。重要的是,提议的计算分析提供了分析分离发生的原因和方式的基本原理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号