当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
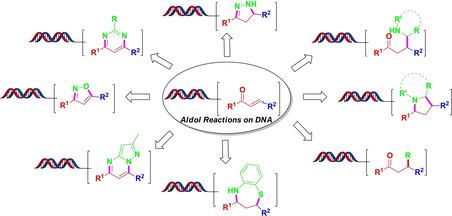
Exploring Aldol Reactions on DNA and Applications to Produce Diverse Structures: An Example of Expanding Chemical Space of DNA‐Encoded Compounds by Diversity‐Oriented Synthesis
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2020-10-29 , DOI: 10.1002/asia.202001105 Rongfeng Wu 1 , Sen Gao 1 , Tian Du 1 , Kunliang Cai 1 , Xuemin Cheng 1 , Jing Fan 1 , Jing Feng 1 , Alex Shaginian 1 , Jin Li 1 , Jinqiao Wan 1 , Guansai Liu 1
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2020-10-29 , DOI: 10.1002/asia.202001105 Rongfeng Wu 1 , Sen Gao 1 , Tian Du 1 , Kunliang Cai 1 , Xuemin Cheng 1 , Jing Fan 1 , Jing Feng 1 , Alex Shaginian 1 , Jin Li 1 , Jinqiao Wan 1 , Guansai Liu 1
Affiliation
|
A DNA‐encoded chemical library (DECL) is built with combinatorial chemistry, which works by bringing chemical fragments together to generate diverse structures. However, chemical diversity of DNA‐encoded chemical libraries is often limited by DNA compatible synthetic reactions. This report shows a conceptual strategy to expand chemical space of DNA‐encoded chemical libraries by incorporation of diversity‐oriented synthesis in DECL synthesis. We developed Aldol reactions on DNA in a combinatorial way. After obtaining DNA‐tagged α, β‐unsaturated ketones which represent important chemical intermediates, many distinct structures with skeletal diversities are achieved by diversity‐oriented synthesis.
中文翻译:

探索有关DNA的Aldol反应及其在生产多样结构中的应用:通过面向多样性的合成扩展DNA编码化合物的化学空间的示例
DNA编码化学文库(DECL)是用组合化学方法构建的,该方法通过将化学片段聚集在一起以生成各种结构来工作。但是,DNA编码的化学文库的化学多样性通常受到DNA兼容的合成反应的限制。该报告显示了通过将面向多样性的合成纳入DECL合成中来扩展DNA编码的化学文库的化学空间的概念性策略。我们以组合方式开发了对DNA的Aldol反应。获得代表重要化学中间体的带有DNA标签的α,β-不饱和酮后,通过面向多样性的合成获得了许多具有骨架多样性的独特结构。
更新日期:2020-12-01
中文翻译:

探索有关DNA的Aldol反应及其在生产多样结构中的应用:通过面向多样性的合成扩展DNA编码化合物的化学空间的示例
DNA编码化学文库(DECL)是用组合化学方法构建的,该方法通过将化学片段聚集在一起以生成各种结构来工作。但是,DNA编码的化学文库的化学多样性通常受到DNA兼容的合成反应的限制。该报告显示了通过将面向多样性的合成纳入DECL合成中来扩展DNA编码的化学文库的化学空间的概念性策略。我们以组合方式开发了对DNA的Aldol反应。获得代表重要化学中间体的带有DNA标签的α,β-不饱和酮后,通过面向多样性的合成获得了许多具有骨架多样性的独特结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号