当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unveiling Electrode–Electrolyte Design-Based NO Reduction for NH3 Synthesis
ACS Energy Letters ( IF 22.0 ) Pub Date : 2020-10-29 , DOI: 10.1021/acsenergylett.0c02082 DongYeon Kim 1 , Dongyup Shin 2 , Juheon Heo 3 , Hyungseob Lim 1 , Jung-Ae Lim 1 , Hyung Mo Jeong 4 , Beom-Sik Kim 1 , Iljeong Heo 1 , Inhwan Oh 1 , Boreum Lee 3 , Monika Sharma 3 , Hankwon Lim 3 , Hyungjun Kim 2 , Youngkook Kwon 3
ACS Energy Letters ( IF 22.0 ) Pub Date : 2020-10-29 , DOI: 10.1021/acsenergylett.0c02082 DongYeon Kim 1 , Dongyup Shin 2 , Juheon Heo 3 , Hyungseob Lim 1 , Jung-Ae Lim 1 , Hyung Mo Jeong 4 , Beom-Sik Kim 1 , Iljeong Heo 1 , Inhwan Oh 1 , Boreum Lee 3 , Monika Sharma 3 , Hankwon Lim 3 , Hyungjun Kim 2 , Youngkook Kwon 3
Affiliation
|
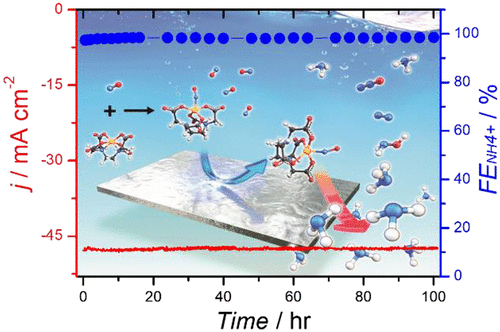
The electrochemical N2 reduction reaction has attracted interest as a potential alternative to the Haber–Bosch process, but a significantly low conversion efficiency and a significantly low ammonia production rate stimulate the need for alternatives. Here, we represent the electrochemical reduction of nitric oxide (NO) on a nanostructured Ag electrode in combination with a rationally designed electrolyte containing the EDTA–Fe2+ metal complex (EFeMC), which results in an ∼100% efficiency for NH3 with a current density of 50 mA/cm2 at −0.165 VRHE, without any degradation in catalytic activity or product selectivity up to 120 h. Economic analysis using itemized cost estimation predicted that the synthesis of ammonia from NO reduction in an EFeMC-designed electrolyte can be market competitive at an electricity price of $0.03 kWh–1 with a current density of >125 mA/cm2. Therefore, this approach opens an entirely new avenue of renewable electricity-driven ammonia synthesis.
中文翻译:

基于电极电解质设计的NH 3合成NO还原技术
电化学N 2还原反应作为哈伯-博世(Haber-Bosch)工艺的潜在替代品引起了人们的兴趣,但是转化效率极低且氨的生产率极低,因此人们需要替代品。在这里,我们表示上的纳米结构的Ag电极的电化学还原一氧化氮(NO)的组合与含有EDTA-铁合理设计的电解质2+金属络合物(EFeMC),其结果在约100%的效率为NH 3与在-0.165 V RHE时的电流密度为50 mA / cm 2长达120小时,催化活性或产物选择性没有任何降低。使用分项成本估算进行的经济分析预测,由EFeMC设计的电解液中的NO还原合成氨气在电价为$ 0.03 kWh –1且电流密度> 125 mA / cm 2时可以具有市场竞争力。因此,这种方法开辟了可再生电力驱动氨合成的全新途径。
更新日期:2020-11-13
中文翻译:

基于电极电解质设计的NH 3合成NO还原技术
电化学N 2还原反应作为哈伯-博世(Haber-Bosch)工艺的潜在替代品引起了人们的兴趣,但是转化效率极低且氨的生产率极低,因此人们需要替代品。在这里,我们表示上的纳米结构的Ag电极的电化学还原一氧化氮(NO)的组合与含有EDTA-铁合理设计的电解质2+金属络合物(EFeMC),其结果在约100%的效率为NH 3与在-0.165 V RHE时的电流密度为50 mA / cm 2长达120小时,催化活性或产物选择性没有任何降低。使用分项成本估算进行的经济分析预测,由EFeMC设计的电解液中的NO还原合成氨气在电价为$ 0.03 kWh –1且电流密度> 125 mA / cm 2时可以具有市场竞争力。因此,这种方法开辟了可再生电力驱动氨合成的全新途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号