当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Study of the Reactions of AlO and OAlO Relevant to Planetary Mesospheres
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-10-29 , DOI: 10.1021/acsearthspacechem.0c00197 Thomas P. Mangan 1 , James M. Harman-Thomas 1 , Rachel E. Lade 1 , Kevin M. Douglas 1 , John M. C. Plane 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-10-29 , DOI: 10.1021/acsearthspacechem.0c00197 Thomas P. Mangan 1 , James M. Harman-Thomas 1 , Rachel E. Lade 1 , Kevin M. Douglas 1 , John M. C. Plane 1
Affiliation
|
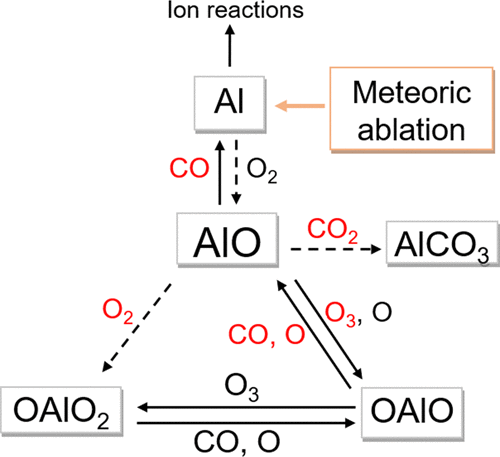
Aluminum atoms are injected into planetary upper atmospheres by meteoric ablation. Rapid oxidation of Al by O2 to form AlO is then likely to be followed by reactions with O3, O2, and CO2 to form larger oxides and carbonates, which can also be reduced by atomic O and CO. The reactions listed below were investigated experimentally using both pulsed laser photolysis of an Al precursor in a slow flow reactor, and pulsed laser ablation of an Al target in a fast flow tube, with laser-induced fluorescence detection of AlO. The experimental results were interpreted using electronic structure theory calculations and Rice–Ramsperger–Kassel–Markus theory. The low pressure limiting rate coefficients for the two recombination reactions are: log10(krec,0 (AlO + O2 + N2, 192–812 K)) = −35.137 + 6.1052 log10(T) – 1.4089 (log10(T))2 and log10(krec,0(AlO + CO2 + N2, 193–813 K)) = −38.736 + 8.7342 log10(T) – 2.0202 (log10(T))2 cm6 molecule–2 s–1, with a ±20% uncertainty over the experimental temperature range. The following bimolecular reactions were also studied at 295 K: k(AlO + O3 → OAlO + O2) = (1.25 ± 0.19) × 10–10; k(AlO + CO → Al + CO2) = (1.95 ± 0.35) × 10–12; k(OAlO + CO → AlO + CO2) = (2.6 ± 0.7) × 10–11 and k(OAlO + O → AlO + O2) = (1.9 ± 0.8) × 10–10 cm3 molecule–1 s–1. In the terrestrial atmosphere between 65 and 110 km, AlO is mostly removed by recombination with O2 below 85 km, and reaction with O3 above 90 km. On Mars, recombination with CO2 is much more important than with O2, although reduction of AlO by CO should maintain a significant density of Al atoms. Here we show that in both atmospheres, AlOH is likely to be an important reservoir.
中文翻译:

AlO和OAlO与行星中间层反应的动力学研究
铝原子通过流平烧蚀注入到行星高层大气中。然后O 2会迅速将Al氧化成AlO,然后与O 3,O 2和CO 2反应形成较大的氧化物和碳酸盐,也可以通过原子O和CO还原。下面列出的反应使用慢流反应器中的Al前驱物进行脉冲激光光解和快速流管中的Al目标的脉冲激光烧蚀,以及激光诱导的AlO荧光检测,进行了实验研究。实验结果用电子结构理论计算和莱斯-拉姆斯伯格-卡塞尔-马库斯理论来解释。两个重组反应的低压极限速率系数为:log10(ķ REC,0(ALO + O 2 + N 2,192-812 K))= -35.137 + 6.1052日志10(Ť) - 1.4089(日志10(Ť))2和日志10(ķ REC,0(的AlO + CO 2 + N 2,193-813 K))= -38.736 + 8.7342日志10(Ť) - 2.0202(日志10(Ť))2厘米6分子-2小号-1,在实验温度范围内具有±20%的不确定度。还研究了在295 K下的以下双分子反应:k(AlO + O 3 →OAlO + O 2)=(1.25±0.19)×10 –10;k(AlO + CO→Al + CO 2)=(1.95±0.35)×10 –12;k(OAlO + CO→AlO + CO 2)=(2.6±0.7)×10 –11,k(OAlO + O→AlO + O 2)=(1.9±0.8)×10 –10 cm 3分子–1 s – 1个。在65至110 km之间的陆地大气中,AlO大部分通过与85 km以下的O 2重组以及与90 km以上的O 3的反应而去除。在火星上,与CO 2的复合比与O 2的复合要重要得多,尽管通过CO还原AlO应该可以保持很大的Al原子密度。在这里,我们表明在两种大气中,AlOH可能是重要的储层。
更新日期:2020-11-19
中文翻译:

AlO和OAlO与行星中间层反应的动力学研究
铝原子通过流平烧蚀注入到行星高层大气中。然后O 2会迅速将Al氧化成AlO,然后与O 3,O 2和CO 2反应形成较大的氧化物和碳酸盐,也可以通过原子O和CO还原。下面列出的反应使用慢流反应器中的Al前驱物进行脉冲激光光解和快速流管中的Al目标的脉冲激光烧蚀,以及激光诱导的AlO荧光检测,进行了实验研究。实验结果用电子结构理论计算和莱斯-拉姆斯伯格-卡塞尔-马库斯理论来解释。两个重组反应的低压极限速率系数为:log10(ķ REC,0(ALO + O 2 + N 2,192-812 K))= -35.137 + 6.1052日志10(Ť) - 1.4089(日志10(Ť))2和日志10(ķ REC,0(的AlO + CO 2 + N 2,193-813 K))= -38.736 + 8.7342日志10(Ť) - 2.0202(日志10(Ť))2厘米6分子-2小号-1,在实验温度范围内具有±20%的不确定度。还研究了在295 K下的以下双分子反应:k(AlO + O 3 →OAlO + O 2)=(1.25±0.19)×10 –10;k(AlO + CO→Al + CO 2)=(1.95±0.35)×10 –12;k(OAlO + CO→AlO + CO 2)=(2.6±0.7)×10 –11,k(OAlO + O→AlO + O 2)=(1.9±0.8)×10 –10 cm 3分子–1 s – 1个。在65至110 km之间的陆地大气中,AlO大部分通过与85 km以下的O 2重组以及与90 km以上的O 3的反应而去除。在火星上,与CO 2的复合比与O 2的复合要重要得多,尽管通过CO还原AlO应该可以保持很大的Al原子密度。在这里,我们表明在两种大气中,AlOH可能是重要的储层。


























 京公网安备 11010802027423号
京公网安备 11010802027423号