当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility of Water in Carbonatites
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-10-29 , DOI: 10.1021/acsearthspacechem.0c00222 Nathan S. Jacobson 1 , Bruce Fegley 2 , John A. Setlock 3 , Gustavo Costa 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-10-29 , DOI: 10.1021/acsearthspacechem.0c00222 Nathan S. Jacobson 1 , Bruce Fegley 2 , John A. Setlock 3 , Gustavo Costa 1
Affiliation
|
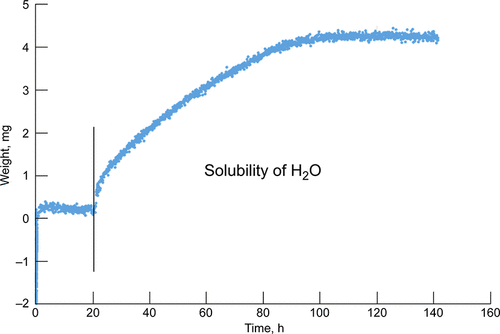
Water solubility in carbonatite magmas at 1 bar total pressure has been explored. Three compositions of carbonatite magma have been studied: natrocarbonatite from Oldoinyo Lengai, Tanzania, and two synthetic compositions CaCO3–Na2CO3–MgCO3 and CaCO3–Na2CO3–CaF2–NaF. All three were characterized with thermal analysis (DSC-TG). Solubility measurements were conducted with a novel microbalance based method adapted from chemical metallurgy. The mixtures were melted under 1 bar of CO2, and then a mixture of H2O/CO2 was admitted. Thus, a direct measurement was made with no effects of cooling such as crystallization and phase changes. The natrocarbonate showed no measurable solubility at 0.57 bar H2O and 800 °C. This is attributed to its altered form from atmosphere exposure, likely compositional changes on weathering. CaCO3–Na2CO3–MgCO3 showed a small solubility (0.47 ± 0.07 wt % H2O at 900 °C and 0.57 bar H2O). This was consistent with an extrapolation from work by other investigators and also additional oxide anions reacting with water molecules in this study. The CaCO3–Na2CO3–CaF2–NaF synthetic magma showed a larger solubility 6.0 ± 1.4 wt % H2O at 0.57 bar H2O and 675 °C. The resultant CaCO3–Na2CO3–CaF2–NaF–H2O melt is described with a Temkin/Regular solution model to understand the dissolution process. The mechanism of dissolution is discussed with particular attention to the effect of fluoride on enhancing water solubility.
中文翻译:

水在碳酸盐岩中的溶解度
已经研究了总压力为1 bar的碳酸岩岩浆中的水溶性。研究了三种碳酸盐岩浆成分:坦桑尼亚Oldoinyo Lengai的钠碳酸盐岩,以及两种合成成分CaCO 3 -Na 2 CO 3 -MgCO 3和CaCO 3 -Na 2 CO 3 -CaF 2 -NaF。通过热分析(DSC-TG)对这三个样品进行了表征。溶解度的测量是采用基于化学冶金的基于微量天平的新型方法进行的。将混合物在1巴的CO 2下熔融,然后在H 2 O / CO 2的混合物下熔融。被录取了。因此,进行了直接测量而没有冷却的影响,例如结晶和相变。碳酸钠在0.57 bar H 2 O和800°C下没有可测量的溶解度。这归因于其暴露于大气中的形式发生了变化,以及风化作用下可能的成分变化。的CaCO 3 -Na 2 CO 3 -MgCO 3显示一个小的溶解度(0.47±0.07重量%H 2 O不连到900℃和0.57巴ħ 2 O)。这与其他研究人员从工作中推断出的结果以及本研究中与水分子反应的其他氧化物阴离子相吻合。CaCO 3 –Na 2 CO 3 –CaF2 -NaF合成岩浆出现较大的溶解度6.0±1.4重量%H 2 O不连到0.57巴ħ 2 O和675℃。用Temkin /常规溶液模型描述了生成的CaCO 3 -Na 2 CO 3 -CaF 2 -NaF-H 2 O熔体,以了解溶解过程。讨论了溶解机理,尤其要注意氟化物对增强水溶性的作用。
更新日期:2020-11-19
中文翻译:

水在碳酸盐岩中的溶解度
已经研究了总压力为1 bar的碳酸岩岩浆中的水溶性。研究了三种碳酸盐岩浆成分:坦桑尼亚Oldoinyo Lengai的钠碳酸盐岩,以及两种合成成分CaCO 3 -Na 2 CO 3 -MgCO 3和CaCO 3 -Na 2 CO 3 -CaF 2 -NaF。通过热分析(DSC-TG)对这三个样品进行了表征。溶解度的测量是采用基于化学冶金的基于微量天平的新型方法进行的。将混合物在1巴的CO 2下熔融,然后在H 2 O / CO 2的混合物下熔融。被录取了。因此,进行了直接测量而没有冷却的影响,例如结晶和相变。碳酸钠在0.57 bar H 2 O和800°C下没有可测量的溶解度。这归因于其暴露于大气中的形式发生了变化,以及风化作用下可能的成分变化。的CaCO 3 -Na 2 CO 3 -MgCO 3显示一个小的溶解度(0.47±0.07重量%H 2 O不连到900℃和0.57巴ħ 2 O)。这与其他研究人员从工作中推断出的结果以及本研究中与水分子反应的其他氧化物阴离子相吻合。CaCO 3 –Na 2 CO 3 –CaF2 -NaF合成岩浆出现较大的溶解度6.0±1.4重量%H 2 O不连到0.57巴ħ 2 O和675℃。用Temkin /常规溶液模型描述了生成的CaCO 3 -Na 2 CO 3 -CaF 2 -NaF-H 2 O熔体,以了解溶解过程。讨论了溶解机理,尤其要注意氟化物对增强水溶性的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号