当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transient Kinetics of O2 Evolution in Photocatalytic Water-Splitting Reaction
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-10-29 , DOI: 10.1021/acscatal.0c04115 Takumu Kosaka 1 , Yuya Teduka 1 , Takuya Ogura 1 , Yuanshu Zhou 2 , Takashi Hisatomi 3 , Hiroshi Nishiyama 4 , Kazunari Domen 3, 4 , Yasufumi Takahashi 2, 5 , Hiroshi Onishi 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-10-29 , DOI: 10.1021/acscatal.0c04115 Takumu Kosaka 1 , Yuya Teduka 1 , Takuya Ogura 1 , Yuanshu Zhou 2 , Takashi Hisatomi 3 , Hiroshi Nishiyama 4 , Kazunari Domen 3, 4 , Yasufumi Takahashi 2, 5 , Hiroshi Onishi 1
Affiliation
|
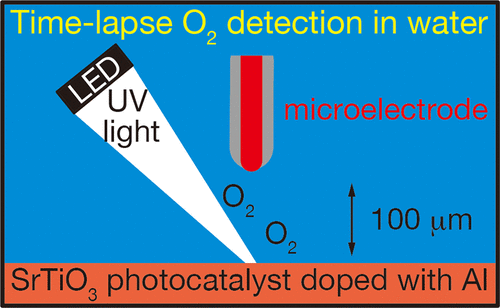
Water splitting to produce H2 and O2 is a fundamental reaction for artificial photosynthesis on semiconductor photocatalysts. The mechanism of the multistepped reaction, especially four-electron oxidation to O2, has not yet been understood. Although some intermediate states have been detected in transient spectroscopy, O2 evolution kinetics remain unknown at the end of consecutive reaction steps. We apply operando O2 detection with a microelectrode to determine the absolute evolution rate on a highly efficient SrTiO3 photocatalyst film casted on a glass plate. The evolution rate was determined with a time resolution of 0.1 s, which was improved by 1000 times compared with that in widely used gas-chromatographic detection. The observed rate did not respond instantaneously to excitation light irradiation. When light was turned on, the photocatalyst film was inactive for evolution and light-activated in seconds. It was proposed that the first absorbed photons were consumed to fill trap states on SrTiO3 surface and then the latter photons drove steady O2 evolution. When excitation light stopped, the O2 evolution rate exponentially decayed in seconds. The microelectrode method demonstrated herein will be useful for understanding many other reaction kinetics at liquid–solid interfaces.
中文翻译:

O 2在光催化水分解反应中的瞬态动力学
分解产生H 2和O 2的水是在半导体光催化剂上进行人工光合作用的基本反应。多步反应的机理,特别是四电子氧化为O 2的机理尚不清楚。尽管在瞬态光谱学中已经检测到一些中间状态,但是在连续反应步骤结束时,O 2的释放动力学仍然未知。我们使用微电极操作O 2检测来确定高效SrTiO 3上的绝对演化速率在玻璃板上浇铸的光触媒膜。用0.1 s的时间分辨率确定析出速率,与广泛使用的气相色谱检测方法相比,析出速率提高了1000倍。观察到的速率没有立即对激发光照射作出响应。当打开灯时,光催化剂膜是无活性的,并在几秒钟内被光激活。提出了先吸收被吸收的光子以填充SrTiO 3表面的陷阱态,然后使后者吸收稳定的O 2逸出。当激发光停止时,O 2进化速度在几秒钟内呈指数下降。本文演示的微电极方法将有助于理解液-固界面处的许多其他反应动力学。
更新日期:2020-11-21
中文翻译:

O 2在光催化水分解反应中的瞬态动力学
分解产生H 2和O 2的水是在半导体光催化剂上进行人工光合作用的基本反应。多步反应的机理,特别是四电子氧化为O 2的机理尚不清楚。尽管在瞬态光谱学中已经检测到一些中间状态,但是在连续反应步骤结束时,O 2的释放动力学仍然未知。我们使用微电极操作O 2检测来确定高效SrTiO 3上的绝对演化速率在玻璃板上浇铸的光触媒膜。用0.1 s的时间分辨率确定析出速率,与广泛使用的气相色谱检测方法相比,析出速率提高了1000倍。观察到的速率没有立即对激发光照射作出响应。当打开灯时,光催化剂膜是无活性的,并在几秒钟内被光激活。提出了先吸收被吸收的光子以填充SrTiO 3表面的陷阱态,然后使后者吸收稳定的O 2逸出。当激发光停止时,O 2进化速度在几秒钟内呈指数下降。本文演示的微电极方法将有助于理解液-固界面处的许多其他反应动力学。


























 京公网安备 11010802027423号
京公网安备 11010802027423号