当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A comparative analysis of GH18 chitinases and their isoforms from Beauveria bassiana: an in-silico approach
Process Biochemistry ( IF 4.4 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.procbio.2020.10.012 Prashant Bhagwat , Ayodeji Amobonye , Suren Singh , Santhosh Pillai
Process Biochemistry ( IF 4.4 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.procbio.2020.10.012 Prashant Bhagwat , Ayodeji Amobonye , Suren Singh , Santhosh Pillai
|
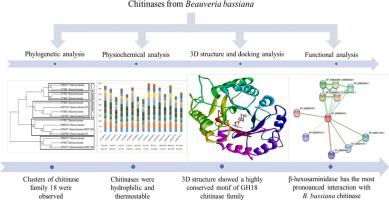
Abstract Fifteen different chitinases from various Beauveria bassiana strains were selected and their physicochemical characteristics, secondary structure evaluation and functional analyses were conducted using multiple bioinformatics tools. The molecular mass of chitinases varied from 34.25 to 49.27 kDa, and theoretical pI from 4.81 to 7.94 respectively. Most of the chitinases were hydrophilic, thermostable and negatively charged with good in vivo half-life. Nearly all the chitinases were extracellular with half of them having the standard secretory peptide, and chitinase family 18 active site motif. Phylogeny, multiple sequence alignment and indel analysis confirmed the presence of highly conserved active site residues in seven sequences. Furthermore, a three-dimensional model of chitinase (AIT18869.1) was constructed using the SWISS-MODEL server and validated by ERRAT, Verify 3D and RAMPAGE. The presence of 98.1 % of its residues in the Ramachandran plot’s favoured region further established the quality of the model. CASTp and MetaPocket 2.0 analysis followed by protein-ligand docking using allosamidin and chitotriose thiazoline, suggested Asp-208, Gln-242, Gln-265 and Asn-268 as the most conserved active residues for the enzyme. The information gathered through the in-silico approach would be beneficial in unravelling the properties of B. bassiana chitinases in vitro, which could be subsequently exploited for various industrial applications.
中文翻译:

球茎白僵菌 GH18 几丁质酶及其同种型的比较分析:一种计算机方法
摘要 从白僵菌中筛选出15种不同的几丁质酶,利用多种生物信息学工具对其理化特性、二级结构评价和功能分析进行了分析。几丁质酶的分子量从 34.25 到 49.27 kDa 不等,理论 pI 分别从 4.81 到 7.94。大多数几丁质酶具有亲水性、热稳定性和负电荷,具有良好的体内半衰期。几乎所有几丁质酶都是细胞外的,其中一半具有标准分泌肽和几丁质酶家族 18 活性位点基序。系统发育、多序列比对和插入缺失分析证实了在七个序列中存在高度保守的活性位点残基。此外,几丁质酶的三维模型(AIT18869. 1) 使用 SWISS-MODEL 服务器构建并通过 ERRAT、Verify 3D 和 RAMPAGE 验证。在拉马钱德兰图的偏爱区域中存在 98.1% 的残基进一步确立了模型的质量。CASTp 和 MetaPocket 2.0 分析,然后使用异氨基丁酸和壳三糖噻唑啉进行蛋白质配体对接,表明 Asp-208、Gln-242、Gln-265 和 Asn-268 是该酶最保守的活性残基。通过计算机内方法收集的信息将有助于在体外解开球孢菌几丁质酶的特性,随后可将其用于各种工业应用。0 分析,然后使用异氨基丁酸和壳三糖噻唑啉进行蛋白质配体对接,表明 Asp-208、Gln-242、Gln-265 和 Asn-268 是该酶最保守的活性残基。通过计算机内方法收集的信息将有助于在体外解开球孢菌几丁质酶的特性,随后可将其用于各种工业应用。0 分析,然后使用异氨基丁酸和壳三糖噻唑啉进行蛋白质配体对接,表明 Asp-208、Gln-242、Gln-265 和 Asn-268 是该酶最保守的活性残基。通过计算机内方法收集的信息将有助于在体外解开球孢菌几丁质酶的特性,随后可将其用于各种工业应用。
更新日期:2021-01-01
中文翻译:

球茎白僵菌 GH18 几丁质酶及其同种型的比较分析:一种计算机方法
摘要 从白僵菌中筛选出15种不同的几丁质酶,利用多种生物信息学工具对其理化特性、二级结构评价和功能分析进行了分析。几丁质酶的分子量从 34.25 到 49.27 kDa 不等,理论 pI 分别从 4.81 到 7.94。大多数几丁质酶具有亲水性、热稳定性和负电荷,具有良好的体内半衰期。几乎所有几丁质酶都是细胞外的,其中一半具有标准分泌肽和几丁质酶家族 18 活性位点基序。系统发育、多序列比对和插入缺失分析证实了在七个序列中存在高度保守的活性位点残基。此外,几丁质酶的三维模型(AIT18869. 1) 使用 SWISS-MODEL 服务器构建并通过 ERRAT、Verify 3D 和 RAMPAGE 验证。在拉马钱德兰图的偏爱区域中存在 98.1% 的残基进一步确立了模型的质量。CASTp 和 MetaPocket 2.0 分析,然后使用异氨基丁酸和壳三糖噻唑啉进行蛋白质配体对接,表明 Asp-208、Gln-242、Gln-265 和 Asn-268 是该酶最保守的活性残基。通过计算机内方法收集的信息将有助于在体外解开球孢菌几丁质酶的特性,随后可将其用于各种工业应用。0 分析,然后使用异氨基丁酸和壳三糖噻唑啉进行蛋白质配体对接,表明 Asp-208、Gln-242、Gln-265 和 Asn-268 是该酶最保守的活性残基。通过计算机内方法收集的信息将有助于在体外解开球孢菌几丁质酶的特性,随后可将其用于各种工业应用。0 分析,然后使用异氨基丁酸和壳三糖噻唑啉进行蛋白质配体对接,表明 Asp-208、Gln-242、Gln-265 和 Asn-268 是该酶最保守的活性残基。通过计算机内方法收集的信息将有助于在体外解开球孢菌几丁质酶的特性,随后可将其用于各种工业应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号