当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Scalable, Telescoped Hydrogenolysis–Enzymatic Decarboxylation Process for the Asymmetric Synthesis of (R)-α-Heteroaryl Propionic Acids
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.oprd.0c00397 Caroline A. Blakemore 1 , Scott P. France 1 , Lacey Samp 2 , Deane M. Nason 1 , Eddie Yang 1 , Roger M. Howard 1 , Karen J. Coffman 1 , Qingyi Yang 3 , Aaron C. Smith 1 , Edelweiss Evrard 3 , Wei Li 4 , Linlin Dai 4 , Lixia Yang 4 , Zhiguang Chen 4 , Qingli Zhang 5 , Fangyan He 5 , Jiesen Zhang 5
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.oprd.0c00397 Caroline A. Blakemore 1 , Scott P. France 1 , Lacey Samp 2 , Deane M. Nason 1 , Eddie Yang 1 , Roger M. Howard 1 , Karen J. Coffman 1 , Qingyi Yang 3 , Aaron C. Smith 1 , Edelweiss Evrard 3 , Wei Li 4 , Linlin Dai 4 , Lixia Yang 4 , Zhiguang Chen 4 , Qingli Zhang 5 , Fangyan He 5 , Jiesen Zhang 5
Affiliation
|
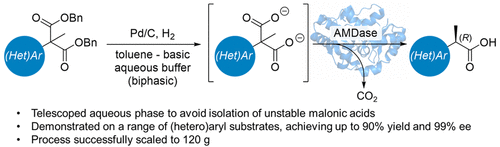
Enantiopure α-aryl propionic acids are useful building blocks for pharmaceutical research and can be accessed enzymatically using arylmalonate decarboxylases (AMDases) from the corresponding malonic acids. However, the intrinsic instability of malonic acids is a major drawback to this approach in which spontaneous decarboxylation can occur, subsequently eroding enantioselectivity and giving rise to racemic products. This was particularly evident for a panel of N-heterocyclic propionic acids that we wished to access using the approach. Herein, we describe a process to overcome the spontaneous decarboxylation problem in which hydrogenolysis of the corresponding dibenzyl malonates was performed in a biphasic toluene–basic aqueous buffer mixture and telescoped into the subsequent AMDase step. This procedure enabled compounds to be accessed in high enantioselectivities and was successfully demonstrated on 120 g with high yield (76%) and ee (98%).
中文翻译:

(R)-α-杂芳基丙酸的不对称合成的可伸缩,伸缩式氢解-酶促脱羧工艺
对映体纯的α-芳基丙酸是药物研究的有用组成部分,可以使用来自相应丙二酸的芳基丙二酸脱羧酶(AMDases)通过酶途径获得。然而,丙二酸的固有不稳定性是该方法的主要缺点,在该方法中,可能发生自发脱羧,随后侵蚀对映选择性并产生外消旋产物。对于我们希望使用该方法获得的一组N-杂环丙酸,这一点尤其明显。在本文中,我们描述了一种克服自发脱羧问题的方法,在该过程中,相应的二苄基丙二酸酯在双相甲苯-碱性水性缓冲液混合物中进行氢解,并伸缩进入随后的AMDase步骤。
更新日期:2020-10-28
中文翻译:

(R)-α-杂芳基丙酸的不对称合成的可伸缩,伸缩式氢解-酶促脱羧工艺
对映体纯的α-芳基丙酸是药物研究的有用组成部分,可以使用来自相应丙二酸的芳基丙二酸脱羧酶(AMDases)通过酶途径获得。然而,丙二酸的固有不稳定性是该方法的主要缺点,在该方法中,可能发生自发脱羧,随后侵蚀对映选择性并产生外消旋产物。对于我们希望使用该方法获得的一组N-杂环丙酸,这一点尤其明显。在本文中,我们描述了一种克服自发脱羧问题的方法,在该过程中,相应的二苄基丙二酸酯在双相甲苯-碱性水性缓冲液混合物中进行氢解,并伸缩进入随后的AMDase步骤。


























 京公网安备 11010802027423号
京公网安备 11010802027423号