当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anchor, Spacer, and Ligand-Modified Engineered Exosomes for Trackable Targeted Therapy
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.bioconjchem.0c00483 Changsun Kang 1 , Patrick Han 2 , Jung S Lee 2 , Dongwon Lee 3 , Dongin Kim 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.bioconjchem.0c00483 Changsun Kang 1 , Patrick Han 2 , Jung S Lee 2 , Dongwon Lee 3 , Dongin Kim 1
Affiliation
|
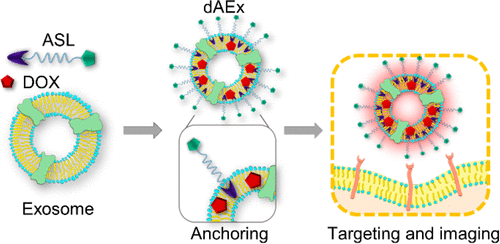
Exosomes have been widely demonstrated as an effective anticancer therapeutic moiety. However, their clinical translation has been limited by the requirement of prohibitively high therapeutic doses due to their lack of specificity in delivery and, consequently, short systemic half-life. To overcome these challenges, we engineered a platform for modifying exosomes with an active targeting modality composed of membrane Anchor (BODIPY)-Spacer (PEG)-targeting Ligands (cyclic RGD peptide) (ASL). Herein, we show that the intramembrane incorporation of a trackable, targeting system renders ASL exosomes (AExs) a modular platform. AExs significantly overcome challenges associated with exosome modification, including potential damage for functionalization, or destabilizing interactions between dyes and drugs. ASL-modification not only enhanced stability in imparting active targeting but also introduced a built-in bioimaging modality. Our studies show that AExs target B16F10 melanoma tumor sites by the specific interaction of cyclic RGD and integrin. Doxorubicin encapsulated AExs (dAExs) significantly inhibited the growth of melanoma in vitro and in vivo. Thus, we conclude that ASL-modification allows exosomes to be transformed into a novel therapeutic vehicle uniquely integrating in vivo tracking and robust targeting with drug delivery. We anticipate that the therapeutic, targeting, and diagnostic modularity provided by ASL will potentiate translational applications of exosome-based vehicles beyond anticancer therapy.
中文翻译:

锚,垫片和配体修饰的工程化外泌体,用于可追踪的靶向治疗
外来体已被广泛证明是有效的抗癌治疗部分。然而,由于其缺乏递送特异性,因此全身半衰期短,因此其临床翻译受到对禁止的高治疗剂量的要求的限制。为了克服这些挑战,我们设计了一个平台,用于修饰具有由膜锚(BODIPY)-间隔物(PEG)-靶向配体(环状RGD肽)(ASL)组成的主动靶向方式的外泌体。在本文中,我们显示可追踪的靶向系统的膜内结合使ASL外泌体(AExs)成为模块化平台。AEx极大地克服了与外泌体修饰相关的挑战,包括功能性破坏或染料与药物之间相互作用的不稳定。ASL修饰不仅增强了主动靶向的稳定性,而且还引入了内置的生物成像技术。我们的研究表明,AEx通过环状RGD和整联蛋白的特异性相互作用靶向B16F10黑色素瘤肿瘤部位。阿霉素包裹的AEx(dAEx)显着抑制黑色素瘤的生长体外和体内。因此,我们得出结论,ASL修饰允许将外泌体转化为独特的整合体内追踪和强大靶向与药物输送的新型治疗载体。我们预计,ASL提供的治疗,靶向和诊断模块将增强基于外来体的载体在抗癌治疗之外的转化应用。
更新日期:2020-11-18
中文翻译:

锚,垫片和配体修饰的工程化外泌体,用于可追踪的靶向治疗
外来体已被广泛证明是有效的抗癌治疗部分。然而,由于其缺乏递送特异性,因此全身半衰期短,因此其临床翻译受到对禁止的高治疗剂量的要求的限制。为了克服这些挑战,我们设计了一个平台,用于修饰具有由膜锚(BODIPY)-间隔物(PEG)-靶向配体(环状RGD肽)(ASL)组成的主动靶向方式的外泌体。在本文中,我们显示可追踪的靶向系统的膜内结合使ASL外泌体(AExs)成为模块化平台。AEx极大地克服了与外泌体修饰相关的挑战,包括功能性破坏或染料与药物之间相互作用的不稳定。ASL修饰不仅增强了主动靶向的稳定性,而且还引入了内置的生物成像技术。我们的研究表明,AEx通过环状RGD和整联蛋白的特异性相互作用靶向B16F10黑色素瘤肿瘤部位。阿霉素包裹的AEx(dAEx)显着抑制黑色素瘤的生长体外和体内。因此,我们得出结论,ASL修饰允许将外泌体转化为独特的整合体内追踪和强大靶向与药物输送的新型治疗载体。我们预计,ASL提供的治疗,靶向和诊断模块将增强基于外来体的载体在抗癌治疗之外的转化应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号