当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
S224 Presents a Catalytic Trade-off in PLP-Dependent l-Lanthionine Synthase from Fusobacterium nucleatum
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.biochem.0c00683 Robert G. Mothersole 1 , Cory R. Billett 1 , Gurpreet Saini 1 , Mina K. Mothersole 1 , Amanda L. Darbyshire 1 , Kirsten R. Wolthers 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-28 , DOI: 10.1021/acs.biochem.0c00683 Robert G. Mothersole 1 , Cory R. Billett 1 , Gurpreet Saini 1 , Mina K. Mothersole 1 , Amanda L. Darbyshire 1 , Kirsten R. Wolthers 1
Affiliation
|
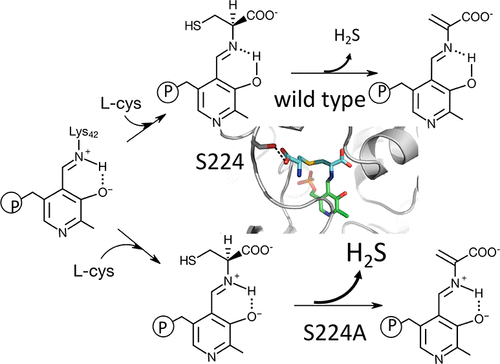
Lanthionine synthase from the oral bacterium Fusobacterium nucleatum is a fold type II pyridoxal-5′-phosphate (PLP)-dependent enzyme that catalyzes the β-replacement of l-cysteine by a second molecule of l-cysteine to form H2S and l-lanthionine. The meso-isomer of the latter product is incorporated into the F. nucleatum peptidoglycan layer. Herein, we investigated the catalytic role of S224, which engages in hydrogen-bond contact with the terminal carboxylate of l-lanthionine in the closed conformation of the enzyme. Unexpectedly, the S224A variant elicited a 7-fold increase in the turnover rate for H2S and lanthionine formation and a 70-fold faster rate constant for the formation of the α-aminoacrylate intermediate compared to the wild-type enzyme. Presteady state kinetic analysis further showed that the reaction between S224A and l-cysteine leads to the formation of the more reactive ketoenamine tautomer of the α-aminoacrylate. The α-aminoacrylate with the protonated Schiff base is not an observable intermediate in the analogous reaction with the wild type, which may account for its attenuated kinetic properties. However, the S224A substitution is detrimental to other aspects of the catalytic cycle; it facilitates the α,β-elimination of l-lanthionine, and it weakens the enzyme’s catalytic preference for the formation of l-lanthionine over that of l-cystathionine.
中文翻译:

S224提出了对来自核梭状芽胞杆菌的PLP依赖的L-蛋氨酸合酶的催化折衷
从口腔细菌羊毛硫氨酸合酶具核梭杆菌为折叠类型II的吡哆醛-5'-磷酸(PLP) -依赖性酶,其催化β-更换升通过的第二分子-半胱氨酸升-半胱氨酸以形成ħ 2 S和升-羊毛硫氨酸。将后者产物的内消旋异构体掺入到核镰状肽肽聚糖层中。在本文中,我们研究了S224的催化作用,该S224在酶的闭合构象中与1-羊毛硫氨酸的末端羧酸酯进行氢键接触。出乎意料的是,S224A变体引起H 2的周转率提高了7倍与野生型酶相比,S和羊毛硫氨酸的形成以及形成α-氨基丙烯酸酯中间体的速率常数快70倍。稳态动力学分析还表明,S224A与1-半胱氨酸之间的反应导致形成了反应性更强的α-氨基丙烯酸酯酮胺互变异构体。在与野生型的类似反应中,带有质子化席夫碱的α-氨基丙烯酸酯不是可观察到的中间体,这可能是其减弱的动力学性质的原因。但是,S224A的取代不利于催化循环的其他方面。它促进了l-羊毛硫氨酸的α,β消除,并且削弱了该酶对l-羊毛硫氨酸形成的催化偏好。l-胱硫醚。
更新日期:2020-11-12
中文翻译:

S224提出了对来自核梭状芽胞杆菌的PLP依赖的L-蛋氨酸合酶的催化折衷
从口腔细菌羊毛硫氨酸合酶具核梭杆菌为折叠类型II的吡哆醛-5'-磷酸(PLP) -依赖性酶,其催化β-更换升通过的第二分子-半胱氨酸升-半胱氨酸以形成ħ 2 S和升-羊毛硫氨酸。将后者产物的内消旋异构体掺入到核镰状肽肽聚糖层中。在本文中,我们研究了S224的催化作用,该S224在酶的闭合构象中与1-羊毛硫氨酸的末端羧酸酯进行氢键接触。出乎意料的是,S224A变体引起H 2的周转率提高了7倍与野生型酶相比,S和羊毛硫氨酸的形成以及形成α-氨基丙烯酸酯中间体的速率常数快70倍。稳态动力学分析还表明,S224A与1-半胱氨酸之间的反应导致形成了反应性更强的α-氨基丙烯酸酯酮胺互变异构体。在与野生型的类似反应中,带有质子化席夫碱的α-氨基丙烯酸酯不是可观察到的中间体,这可能是其减弱的动力学性质的原因。但是,S224A的取代不利于催化循环的其他方面。它促进了l-羊毛硫氨酸的α,β消除,并且削弱了该酶对l-羊毛硫氨酸形成的催化偏好。l-胱硫醚。



























 京公网安备 11010802027423号
京公网安备 11010802027423号