当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of Engineered Ferredoxin Reductase Systems for the Efficient Hydroxylation of Steroidal Substrates
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-10-28 , DOI: 10.1021/acssuschemeng.0c07042 Zhangliang Zhu 1 , Xin Gao 1 , Zhan Song 1 , Chao Li 1 , Fuping Lu 1 , Masaru Tanokura 1, 2 , Hui-Min Qin 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-10-28 , DOI: 10.1021/acssuschemeng.0c07042 Zhangliang Zhu 1 , Xin Gao 1 , Zhan Song 1 , Chao Li 1 , Fuping Lu 1 , Masaru Tanokura 1, 2 , Hui-Min Qin 1
Affiliation
|
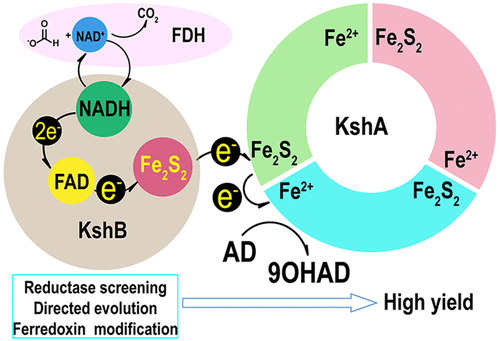
9α-Hydroxy-4-androstene-3,17-dione (9OHAD), formed by the 9α-hydroxylation reaction catalyzed by 3-ketosteroid-9-hydroxylase (KSH), is an important precursor for the synthesis of adrenocortical hormones. KSH, a key enzyme complex in microbial steroid catabolism, contains a Rieske oxygenase (KshA) and a ferredoxin reductase (KshB). In this study, first we determined that the activity of KshB was rate-limiting in 9OHAD production. Thus, several potential alternative reductases were screened; a toluene 2,3-dioxygenase (TDO) reductase showed the highest activity toward NADH. In pathway optimization, the addition of a Rieske [2Fe–2S] cluster to KshB or TDO resulted in improved 9OHAD yields, which implies an improved efficiency of electron transfer. A sufficient supply system of NADH was ensured by introducing formate dehydrogenase (FDH) to construct an NADH regeneration system. The biosynthesis of 9OHAD was then optimized in a whole Escherichia coli cell catalysis system expressing FDH, KshA, and a variant of TDO reductase containing five point mutations and an added Rieske [2Fe–2S] cluster, which resulted in the final production of 5.24 g/L 9OHAD from 4-androstene-3,17-dione with 99.3% yield. This research provides detailed insight into the electron-transfer system for steroid hydroxylation reactions.
中文翻译:

工程化铁氧还蛋白还原酶系统的开发,可有效甾体基质的羟化反应
由3-酮类固醇9-羟化酶(KSH)催化的9α-羟基化反应形成的9α-羟基-4-雄烯基3,17-二酮(9OHAD)是肾上腺皮质激素合成的重要前体。KSH是微生物类固醇分解代谢中的关键酶复合物,包含Rieske加氧酶(KshA)和铁氧还蛋白还原酶(KshB)。在这项研究中,我们首先确定KshB的活性在9OHAD生产中是限速的。因此,筛选了几种潜在的替代还原酶。甲苯2,3-二加氧酶(TDO)还原酶对NADH的活性最高。在途径优化中,向KshB或TDO添加Rieske [2Fe–2S]簇可以提高9OHAD的产率,这意味着电子转移的效率得到提高。通过引入甲酸脱氢酶(FDH)来构建NADH再生系统,确保了NADH的充足供应系统。然后整体上优化了9OHAD的生物合成表达FDH,KshA和TDO还原酶变体的大肠杆菌细胞催化系统,该变体包含五个点突变和一个附加的Rieske [2Fe–2S]簇,最终从4-雄烯-3产生5.24 g / L 9OHAD, 17-二酮,产率99.3%。这项研究为类固醇羟基化反应的电子转移系统提供了详细的见识。
更新日期:2020-11-09
中文翻译:

工程化铁氧还蛋白还原酶系统的开发,可有效甾体基质的羟化反应
由3-酮类固醇9-羟化酶(KSH)催化的9α-羟基化反应形成的9α-羟基-4-雄烯基3,17-二酮(9OHAD)是肾上腺皮质激素合成的重要前体。KSH是微生物类固醇分解代谢中的关键酶复合物,包含Rieske加氧酶(KshA)和铁氧还蛋白还原酶(KshB)。在这项研究中,我们首先确定KshB的活性在9OHAD生产中是限速的。因此,筛选了几种潜在的替代还原酶。甲苯2,3-二加氧酶(TDO)还原酶对NADH的活性最高。在途径优化中,向KshB或TDO添加Rieske [2Fe–2S]簇可以提高9OHAD的产率,这意味着电子转移的效率得到提高。通过引入甲酸脱氢酶(FDH)来构建NADH再生系统,确保了NADH的充足供应系统。然后整体上优化了9OHAD的生物合成表达FDH,KshA和TDO还原酶变体的大肠杆菌细胞催化系统,该变体包含五个点突变和一个附加的Rieske [2Fe–2S]簇,最终从4-雄烯-3产生5.24 g / L 9OHAD, 17-二酮,产率99.3%。这项研究为类固醇羟基化反应的电子转移系统提供了详细的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号