当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
De Novo Biosynthesis of Multiple Pinocembrin Derivatives in Saccharomyces cerevisiae
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-10-27 , DOI: 10.1021/acssynbio.0c00289 Xiaonan Liu 1, 2 , Jian Cheng 1 , Xiaoxi Zhu 1, 2 , Guanghui Zhang 3 , Shengchao Yang 3 , Xiaoxian Guo 1 , Huifeng Jiang 1 , Yanhe Ma 1
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-10-27 , DOI: 10.1021/acssynbio.0c00289 Xiaonan Liu 1, 2 , Jian Cheng 1 , Xiaoxi Zhu 1, 2 , Guanghui Zhang 3 , Shengchao Yang 3 , Xiaoxian Guo 1 , Huifeng Jiang 1 , Yanhe Ma 1
Affiliation
|
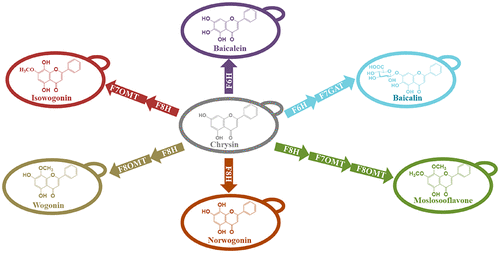
Pinocembrin derived flavones are the major bioactive compounds presented in the Lamiaceae plants that have long been of interest due to their great pharmaceutical and economical significance. Modifications on the central skeleton of the flavone moiety have a huge impact on their biological activities. However, the enzymes responsible for structure modification of most flavones are either inefficient or remain unidentified. By integrating omics analysis of Scutellaria barbata and synthetic biology tools in yeast chassis, we characterized a novel gene encoding flavone 7-O-methyltransferase (F7OMT) and discovered a new flavone 8-hydroxylase (F8H) with increased activity. We also identified a series of flavone 6-hydroxylases (F6Hs) and flavone 8-O-methyltransferases (F8OMTs) in this study. Subsequently, we constructed the biosynthetic pathway for chrysin production by assembling catalytic elements from different species and improved the titer to 10.06 mg/L. Using the established chrysin production platform, we achieved the de novo biosynthesis of baicalein, baicalin, norwogonin, wogonin, isowogonin, and moslosooflavone in yeast. Our results indicated that the combination of omics and synthetic biology can greatly speed up the efficiency of gene mining in plants and the engineered yeasts established an alternative way for the production of pinocembrin derivatives.
中文翻译:

酿酒酵母中多种松柏素衍生物的从头生物合成
Pinocembrin 衍生的黄酮是唇形科植物中存在的主要生物活性化合物,由于其巨大的药学和经济意义,长期以来一直受到关注。黄酮部分中心骨架的修饰对其生物活性有巨大影响。然而,负责大多数黄酮结构修饰的酶要么效率低下,要么仍未确定。通过在酵母底盘中整合Scutellaria barbata 的组学分析和合成生物学工具,我们表征了一种编码黄酮 7- O-甲基转移酶 (F7OMT)的新基因,并发现了一种活性增加的新黄酮 8-羟化酶 (F8H)。我们还鉴定了一系列黄酮 6-羟化酶 (F6Hs) 和黄酮 8- O-甲基转移酶 (F8OMTs) 在本研究中。随后,我们通过组装来自不同物种的催化元素构建了白杨素生产的生物合成途径,并将滴度提高到 10.06 mg/L。利用已建立的白杨素生产平台,我们在酵母中实现了黄芩素、黄芩苷、去甲黄芩素、汉黄芩素、异黄芩苷和moslosooflavone的从头生物合成。我们的研究结果表明,组学和合成生物学的结合可以大大加快植物基因挖掘的效率,工程酵母为生产松柏素衍生物建立了另一种途径。
更新日期:2020-11-21
中文翻译:

酿酒酵母中多种松柏素衍生物的从头生物合成
Pinocembrin 衍生的黄酮是唇形科植物中存在的主要生物活性化合物,由于其巨大的药学和经济意义,长期以来一直受到关注。黄酮部分中心骨架的修饰对其生物活性有巨大影响。然而,负责大多数黄酮结构修饰的酶要么效率低下,要么仍未确定。通过在酵母底盘中整合Scutellaria barbata 的组学分析和合成生物学工具,我们表征了一种编码黄酮 7- O-甲基转移酶 (F7OMT)的新基因,并发现了一种活性增加的新黄酮 8-羟化酶 (F8H)。我们还鉴定了一系列黄酮 6-羟化酶 (F6Hs) 和黄酮 8- O-甲基转移酶 (F8OMTs) 在本研究中。随后,我们通过组装来自不同物种的催化元素构建了白杨素生产的生物合成途径,并将滴度提高到 10.06 mg/L。利用已建立的白杨素生产平台,我们在酵母中实现了黄芩素、黄芩苷、去甲黄芩素、汉黄芩素、异黄芩苷和moslosooflavone的从头生物合成。我们的研究结果表明,组学和合成生物学的结合可以大大加快植物基因挖掘的效率,工程酵母为生产松柏素衍生物建立了另一种途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号