当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molten-Salt-Protected Pyrolysis for Fabricating Perovskite Nanocrystals with Promoted Water Oxidation Behavior
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-10-27 , DOI: 10.1021/acssuschemeng.0c06971 Yanqing Guo 1 , Fengcai Lei 1 , Jindi Qi 1 , Shanshan Cao 1 , Zimeng Wei 1 , Shanshan Lou 1 , Pin Hao 1 , Junfeng Xie 1 , Bo Tang 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-10-27 , DOI: 10.1021/acssuschemeng.0c06971 Yanqing Guo 1 , Fengcai Lei 1 , Jindi Qi 1 , Shanshan Cao 1 , Zimeng Wei 1 , Shanshan Lou 1 , Pin Hao 1 , Junfeng Xie 1 , Bo Tang 1
Affiliation
|
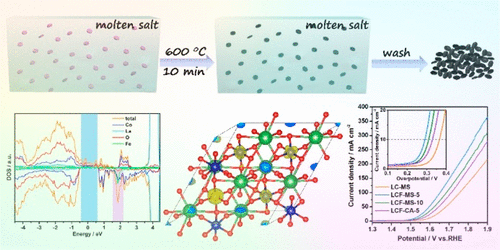
The high overpotential for triggering the oxygen evolution reaction (OER) severely hampers promotion of the overall efficiency for water electrolysis, which significantly restricts implementation of commercial electrocatalytic hydrogen production. Toward exploring advanced OER catalysts with both high efficiency and low cost, transition metal compounds have been regarded as promising alternatives to replace precious metal-based catalysts, among which the cobalt-based materials, especially LaCoO3 perovskites, are highly attractive owing to their tunable electronic structure, relatively high activity, and superior stability. However, the harsh reaction environments for the fabrication of perovskites often result in micrometer-scale particles with limited surface sites, and modulation of electronic structures is also required to be further optimized. In this work, we proposed a molten-salt-protected pyrolysis (MSPP) route to convert the amorphous nanoparticle precursors to LaCoO3 nanocrystals with tunable Fe doping, during which the molten salts could not only act as an effective reaction medium to avoid interparticle sintering but also induce enrichment of surface Co3+ ions with high catalytic activity. Theoretical and experimental analyses indicate that Fe doping could significantly modulate the electronic structure of LaCoO3, resulting in enhanced Co–O covalency and facile charge transfer behavior during the OER. With the above merits, remarkable OER performance with ultralow overpotential, high catalytic current density, small Tafel slope, outstanding intrinsic OER activity, and superior operational stability can be synergistically achieved for the Fe-doped LaCoO3 nanocrystals, making the perovskite nanocatalyst a promising candidate for electrochemical water splitting.
中文翻译:

熔融盐保护热解法制备具有促进水氧化行为的钙钛矿纳米晶体
用于触发氧释放反应(OER)的高过电势严重阻碍了水电解总效率的提高,这极大地限制了商业电催化制氢的实施。为了探索既高效又低成本的先进OER催化剂,过渡金属化合物已被视为替代贵金属基催化剂的有前途的替代品,其中包括钴基材料,尤其是LaCoO 3钙钛矿由于其可调的电子结构,相对较高的活性和出色的稳定性而极具吸引力。然而,用于制造钙钛矿的苛刻反应环境经常导致具有有限表面位点的微米级颗粒,并且还需要进一步优化电子结构的调制。在这项工作中,我们提出了一种熔融盐保护的热解(MSPP)路线,以可调谐的Fe掺杂将无定形的纳米颗粒前体转化为LaCoO 3纳米晶体,在此过程中,熔融盐不仅可以充当有效的反应介质,避免颗粒间烧结但也会引起表面Co 3+的富集离子具有高催化活性。理论和实验分析表明,Fe掺杂可以显着调节LaCoO 3的电子结构,从而导致OER过程中Co-O价增强,电荷转移行为容易。凭借以上优点,Fe掺杂的LaCoO 3纳米晶体可协同获得超低电势,超低的超电势,高的催化电流密度,小的Tafel斜率,出色的固有OER活性和优异的操作稳定性,这使得钙钛矿纳米催化剂成为有希望的候选物用于电化学水分解。
更新日期:2020-11-09
中文翻译:

熔融盐保护热解法制备具有促进水氧化行为的钙钛矿纳米晶体
用于触发氧释放反应(OER)的高过电势严重阻碍了水电解总效率的提高,这极大地限制了商业电催化制氢的实施。为了探索既高效又低成本的先进OER催化剂,过渡金属化合物已被视为替代贵金属基催化剂的有前途的替代品,其中包括钴基材料,尤其是LaCoO 3钙钛矿由于其可调的电子结构,相对较高的活性和出色的稳定性而极具吸引力。然而,用于制造钙钛矿的苛刻反应环境经常导致具有有限表面位点的微米级颗粒,并且还需要进一步优化电子结构的调制。在这项工作中,我们提出了一种熔融盐保护的热解(MSPP)路线,以可调谐的Fe掺杂将无定形的纳米颗粒前体转化为LaCoO 3纳米晶体,在此过程中,熔融盐不仅可以充当有效的反应介质,避免颗粒间烧结但也会引起表面Co 3+的富集离子具有高催化活性。理论和实验分析表明,Fe掺杂可以显着调节LaCoO 3的电子结构,从而导致OER过程中Co-O价增强,电荷转移行为容易。凭借以上优点,Fe掺杂的LaCoO 3纳米晶体可协同获得超低电势,超低的超电势,高的催化电流密度,小的Tafel斜率,出色的固有OER活性和优异的操作稳定性,这使得钙钛矿纳米催化剂成为有希望的候选物用于电化学水分解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号