Chemical & Pharmaceutical Bulletin ( IF 1.7 ) Pub Date : 2020-10-01 , DOI: 10.1248/cpb.c20-00178 Hiromichi Fujioka 1
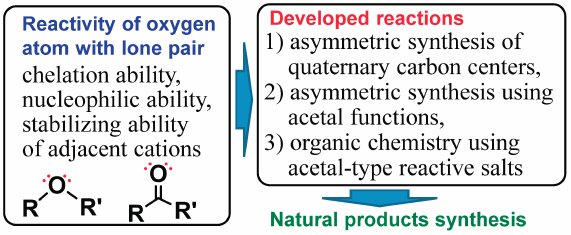
Oxygen atoms have a lone pair of electrons, so they have high chelation ability, high nucleophilic ability, stabilizing ability of adjacent cations, and take a chelate or oxocarbenium ion structure with Lewis acids and metals. I took advantage of these properties to develop three new reactions, 1) asymmetric synthesis of chiral quaternary carbon centers, 2) asymmetric synthesis using acetal functions, and 3) organic chemistry using acetal-type reactive salt chemical species, and applied them to biologically active natural products synthesis. New reactions described here are all innovative and useful for natural products synthesis. In particular, the first asymmetric synthesis of fredericamycin A, and concise asymmetric synthesis of anthracycline antibiotics, scyphostatin, (+)-Sch 642305, (−)-stenine, clavolonine, (+)-rubrenolide, (+)-rubrynolide, (+)-centrolobine, and decytospolide A and B, etc., are noteworthy. Furthermore, since reactions using acetal-type reactive salt chemical species allow the coexistence of functional groups that normally cannot coexist, the reactions using reactive salts have potential to change the retrosynthesis planned based on conventional reactions.
 Graphical Abstract Fullsize Image
Graphical Abstract Fullsize Image
中文翻译:

孤对氧原子对新型创新合成有机化学的发展
氧原子具有一对孤电子,因此它们具有高螯合能力,高亲核能力,相邻阳离子的稳定能力,并与路易斯酸和金属形成螯合或氧碳鎓离子结构。我利用这些性质开发了三个新反应:1)手性季碳中心的不对称合成,2)使用乙缩醛功能的不对称合成,以及3)使用缩醛型反应性盐化学物种的有机化学,并将其应用于生物活性天然产物合成。此处描述的新反应都是创新的,可用于天然产物的合成。尤其是首次合成非弗雷霉素霉素A和简明不对称合成蒽环类抗生素,鞘氨醇抑素,(+)-Sch 642305,(-)-斯汀宁,clavolonine,(+)-丁香内酯,(+)-丁香内酯,等,值得注意。此外,由于使用缩醛型反应性盐化学物质的反应允许通常不能共存的官能团的共存,因此使用反应性盐的反应具有改变基于常规反应计划的逆合成的潜力。
 图形抽象全尺寸图像
图形抽象全尺寸图像


























 京公网安备 11010802027423号
京公网安备 11010802027423号