当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the copper binding ability of Mets7 hCtr‐1 protein domain and His7 derivative: An insight in Michael addition catalysis
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2020-10-22 , DOI: 10.1002/psc.3289 Isabella Rimoldi 1 , Raffaella Bucci 1 , Lucia Feni 1 , Laura Santagostini 2 , Giorgio Facchetti 1 , Sara Pellegrino 1
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2020-10-22 , DOI: 10.1002/psc.3289 Isabella Rimoldi 1 , Raffaella Bucci 1 , Lucia Feni 1 , Laura Santagostini 2 , Giorgio Facchetti 1 , Sara Pellegrino 1
Affiliation
|
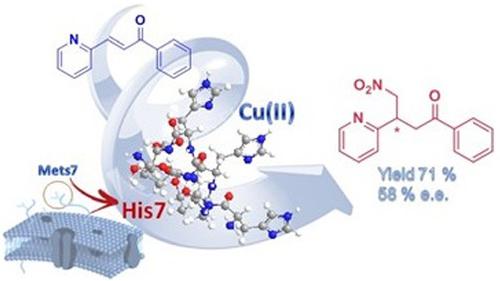
Mets7 is a methionine‐rich motif present in hCtr‐1 transporter that is involved in copper cellular trafficking. Its ability to bind Cu(I) was recently exploited to develop metallopeptide catalysts for Henry condensation. Here, the catalytic activity of Mets7‐Cu(I) complex in Michael addition reactions has been evaluated. Furthermore, His7 peptide, in which Met residues have been substituted with His ones, was also prepared. This substitution allowed His7 to coordinate Cu (II), with the obtainment of a stable turn conformation as evicted by CD experiments. His7‐Cu (II) proved also to be a better catalyst than Mets7‐Cu(I) in the addition reaction. In particular, when the substrate was the (E)‐1‐phenyl‐3‐(pyridin‐2‐yl)prop‐2‐en‐1‐one, a conversion of 71% and a significative 58% of e.e. was observed.
中文翻译:

探索 Mets7 hCtr-1 蛋白结构域和 His7 衍生物的铜结合能力:对迈克尔加成催化的洞察
Mets7是存在于 hCtr-1 转运蛋白中的富含甲硫氨酸的基序,参与铜细胞运输。它结合 Cu(I) 的能力最近被用来开发用于亨利缩合的金属肽催化剂。在这里,已经评估了Mets7-Cu(I) 配合物在迈克尔加成反应中的催化活性。此外,还制备了其中Met残基已被His残基取代的His7肽。这种取代允许His7与 Cu (II) 配位,获得了稳定的转角构象,如 CD 实验所驱逐。在加成反应中,His7-Cu (II)也被证明是比Mets7-Cu(I)更好的催化剂。特别是,当底物是 (E )-1-苯基-3-(吡啶-2-基)prop-2-en-1-one,观察到71%的转化率和58%的ee转化率。
更新日期:2020-10-22
中文翻译:

探索 Mets7 hCtr-1 蛋白结构域和 His7 衍生物的铜结合能力:对迈克尔加成催化的洞察
Mets7是存在于 hCtr-1 转运蛋白中的富含甲硫氨酸的基序,参与铜细胞运输。它结合 Cu(I) 的能力最近被用来开发用于亨利缩合的金属肽催化剂。在这里,已经评估了Mets7-Cu(I) 配合物在迈克尔加成反应中的催化活性。此外,还制备了其中Met残基已被His残基取代的His7肽。这种取代允许His7与 Cu (II) 配位,获得了稳定的转角构象,如 CD 实验所驱逐。在加成反应中,His7-Cu (II)也被证明是比Mets7-Cu(I)更好的催化剂。特别是,当底物是 (E )-1-苯基-3-(吡啶-2-基)prop-2-en-1-one,观察到71%的转化率和58%的ee转化率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号