当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of Phosphopeptide Positional Isomers on the Transcriptional Co-activator TAZ
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.biochem.0c00521 Justin Roberto 1 , Catherine E. Sykes 1 , Panayiotis O. Vacratsis 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.biochem.0c00521 Justin Roberto 1 , Catherine E. Sykes 1 , Panayiotis O. Vacratsis 1
Affiliation
|
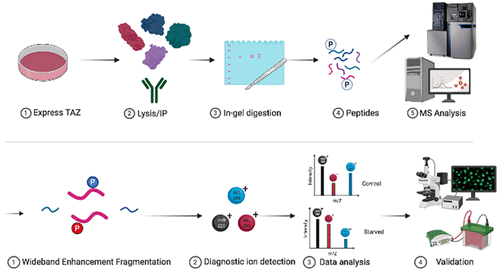
The transcriptional co-activator with the PDZ binding motif (TAZ) is a critical regulator of numerous cellular processes such as cell differentiation, development, proliferation, and cell growth. Aberrant expression and activity of TAZ are also featured in many human malignancies. A hallmark of TAZ biology is its cytoplasmic retention mediated by 14-3-3 isoforms in response to phosphorylation of Ser89 by members of the LATS family of kinases. Following the observation that TAZ is a highly phosphorylated protein even when Ser89 is mutated, high-resolution mass spectrometry employing data-independent acquisition and ion mobility separation was conducted to elucidate additional TAZ phosphorylation sites that may play a role in regulating this critical transcriptional rheostat. Numerous phosphorylation sites on TAZ were identified, including several novel modifications. Of notable interest was the identification of positional phosphoisomers on a phosphopeptide containing Ser89. Optimized use of a so-called wideband enhancement acquisition technique yielded higher-quality fragmentation data that confirmed the detection of Ser93 as the positional phosphoisomer partner of Ser89 and identified diagnostic fragment ions for the phosphorylation events. Functional analysis indicated that Ser93 phosphorylation reduces the level of 14-3-3 association and increases the level of nuclear translocation, indicating this phosphorylation event attenuates the 14-3-3-mediated TAZ cytoplasmic retention mechanism. These findings suggest that the biological activities of TAZ are likely dynamically regulated by multisite phosphorylation.
中文翻译:

转录共激活物TAZ上的磷酸肽位置异构体的表征
具有PDZ结合基序(TAZ)的转录共激活因子是许多细胞过程(例如细胞分化,发育,增殖和细胞生长)的关键调节剂。TAZ的异常表达和活性也存在于许多人类恶性肿瘤中。TAZ生物学的一个标志是它的细胞质保留是由LATS家族的成员响应Ser 89的磷酸化而由14-3-3亚型介导的。观察到TAZ是高度磷酸化的蛋白质,即使Ser 89突变的高分辨率质谱仪采用独立于数据的采集,并进行了离子迁移分离,以阐明可能在调节此关键转录变阻剂中起作用的其他TAZ磷酸化位点。鉴定了TAZ上的许多磷酸化位点,包括几种新颖的修饰。值得注意的是在含有Ser 89的磷酸肽上鉴定位置磷酸异构体。优化使用的所谓的宽带增强采集技术的产生该确认丝氨酸的检测更高质量的碎片数据93作为序列的位置phosphoisomer伙伴89和识别的诊断片段离子的磷酸化事件。功能分析表明93磷酸化可降低14-3-3缔合的水平并增加核易位的水平,表明该磷酸化事件减弱了14-3-3-介导的TAZ细胞质保留机制。这些发现表明,TAZ的生物活性可能受多位磷酸化的动态调节。
更新日期:2020-11-03
中文翻译:

转录共激活物TAZ上的磷酸肽位置异构体的表征
具有PDZ结合基序(TAZ)的转录共激活因子是许多细胞过程(例如细胞分化,发育,增殖和细胞生长)的关键调节剂。TAZ的异常表达和活性也存在于许多人类恶性肿瘤中。TAZ生物学的一个标志是它的细胞质保留是由LATS家族的成员响应Ser 89的磷酸化而由14-3-3亚型介导的。观察到TAZ是高度磷酸化的蛋白质,即使Ser 89突变的高分辨率质谱仪采用独立于数据的采集,并进行了离子迁移分离,以阐明可能在调节此关键转录变阻剂中起作用的其他TAZ磷酸化位点。鉴定了TAZ上的许多磷酸化位点,包括几种新颖的修饰。值得注意的是在含有Ser 89的磷酸肽上鉴定位置磷酸异构体。优化使用的所谓的宽带增强采集技术的产生该确认丝氨酸的检测更高质量的碎片数据93作为序列的位置phosphoisomer伙伴89和识别的诊断片段离子的磷酸化事件。功能分析表明93磷酸化可降低14-3-3缔合的水平并增加核易位的水平,表明该磷酸化事件减弱了14-3-3-介导的TAZ细胞质保留机制。这些发现表明,TAZ的生物活性可能受多位磷酸化的动态调节。



























 京公网安备 11010802027423号
京公网安备 11010802027423号