当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cross-validated Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging Quantitation Protocol for a Pharmaceutical Drug and Its Drug-Target Effects in the Brain Using Time-of-Flight and Fourier Transform Ion Cyclotron Resonance Analyzers
Analytical Chemistry ( IF 7.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.analchem.0c03203 Patrik Källback 1 , Theodosia Vallianatou 1 , Anna Nilsson 1, 2 , Reza Shariatgorji 1, 2 , Nicoletta Schintu 3 , Marcela Pereira 3 , Florian Barré 1 , Henrik Wadensten 1 , Per Svenningsson 3 , Per E. Andrén 1, 2
Analytical Chemistry ( IF 7.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.analchem.0c03203 Patrik Källback 1 , Theodosia Vallianatou 1 , Anna Nilsson 1, 2 , Reza Shariatgorji 1, 2 , Nicoletta Schintu 3 , Marcela Pereira 3 , Florian Barré 1 , Henrik Wadensten 1 , Per Svenningsson 3 , Per E. Andrén 1, 2
Affiliation
|
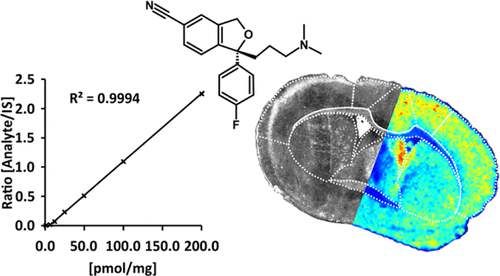
Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) is an established tool in drug development, which enables visualization of drugs and drug metabolites at spatial localizations in tissue sections from different organs. However, robust and accurate quantitation by MALDI-MSI still remains a challenge. We present a quantitative MALDI-MSI method using two instruments with different types of mass analyzers, i.e., time-of-flight (TOF) and Fourier transform ion cyclotron resonance (FTICR) MS, for mapping levels of the in vivo-administered drug citalopram, a selective serotonin reuptake inhibitor, in mouse brain tissue sections. Six different methods for applying calibration standards and an internal standard were evaluated. The optimized method was validated according to authorities’ guidelines and requirements, including selectivity, accuracy, precision, recovery, calibration curve, sensitivity, reproducibility, and stability parameters. We showed that applying a dilution series of calibration standards followed by a homogeneously applied, stable, isotopically labeled standard for normalization and a matrix on top of the tissue section yielded similar results to those from the reference method using liquid chromatography–tandem mass spectrometry (LC–MS/MS). The validation results were within specified limits and the brain concentrations for TOF MS (51.1 ± 4.4 pmol/mg) and FTICR MS (56.9 ± 6.0 pmol/mg) did not significantly differ from those of the cross-validated LC–MS/MS method (55.0 ± 4.9 pmol/mg). The effect of in vivo citalopram administration on the serotonin neurotransmitter system was studied in the hippocampus, a brain region that is the principal target of the serotonergic afferents along with the limbic system, and it was shown that serotonin was significantly increased (2-fold), but its metabolite 5-hydroxyindoleacetic acid was not. This study makes a substantial step toward establishing MALDI-MSI as a fully quantitative validated method.
中文翻译:

使用飞行时间和傅立叶变换离子回旋共振分析仪对药物及其在大脑中的药物靶效应进行交叉验证的基质辅助激光解吸/电离质谱成像定量方案
基质辅助激光解吸/电离质谱成像(MALDI-MSI)是药物开发中已建立的工具,它可以使不同器官的组织切片中空间定位的药物和药物代谢物可视化。然而,通过MALDI-MSI进行可靠,准确的定量分析仍然是一个挑战。我们提出了使用两种仪器和不同类型的质量分析仪(即飞行时间(TOF)和傅里叶变换离子回旋共振(FTICR)MS)的定量MALDI-MSI方法,用于绘制体内水平在小鼠脑组织切片中使用西酞普兰(一种选择性的5-羟色胺再摄取抑制剂)。评估了六种不同的校准标准品和内标的应用方法。该优化方法已根据有关部门的指导原则和要求进行了验证,包括选择性,准确性,精密度,回收率,校准曲线,灵敏度,重现性和稳定性参数。我们发现,应用一系列稀释的校准标准品,然后再进行均一的,稳定的,同位素标记的标准品进行标准化,并在组织切片顶部添加基质,其结果与液相色谱-串联质谱法(LC)的参考方法相似。 –MS / MS)。验证结果在规定的范围内,TOF MS的大脑浓度为(51.1±4)。4 pmol / mg)和FTICR MS(56.9±6.0 pmol / mg)与交叉验证的LC-MS / MS方法(55.0±4.9 pmol / mg)并无显着差异。的效果在海马体中研究了西酞普兰对5-羟色胺神经递质系统的体内给药,海马是5-羟色胺能传入神经和边缘系统的主要靶点的大脑区域,结果显示5-羟色胺显着增加(2倍),但代谢产物不是5-羟基吲哚乙酸。这项研究朝着建立MALDI-MSI作为完全定量验证的方法迈出了实质性的一步。
更新日期:2020-11-03
中文翻译:

使用飞行时间和傅立叶变换离子回旋共振分析仪对药物及其在大脑中的药物靶效应进行交叉验证的基质辅助激光解吸/电离质谱成像定量方案
基质辅助激光解吸/电离质谱成像(MALDI-MSI)是药物开发中已建立的工具,它可以使不同器官的组织切片中空间定位的药物和药物代谢物可视化。然而,通过MALDI-MSI进行可靠,准确的定量分析仍然是一个挑战。我们提出了使用两种仪器和不同类型的质量分析仪(即飞行时间(TOF)和傅里叶变换离子回旋共振(FTICR)MS)的定量MALDI-MSI方法,用于绘制体内水平在小鼠脑组织切片中使用西酞普兰(一种选择性的5-羟色胺再摄取抑制剂)。评估了六种不同的校准标准品和内标的应用方法。该优化方法已根据有关部门的指导原则和要求进行了验证,包括选择性,准确性,精密度,回收率,校准曲线,灵敏度,重现性和稳定性参数。我们发现,应用一系列稀释的校准标准品,然后再进行均一的,稳定的,同位素标记的标准品进行标准化,并在组织切片顶部添加基质,其结果与液相色谱-串联质谱法(LC)的参考方法相似。 –MS / MS)。验证结果在规定的范围内,TOF MS的大脑浓度为(51.1±4)。4 pmol / mg)和FTICR MS(56.9±6.0 pmol / mg)与交叉验证的LC-MS / MS方法(55.0±4.9 pmol / mg)并无显着差异。的效果在海马体中研究了西酞普兰对5-羟色胺神经递质系统的体内给药,海马是5-羟色胺能传入神经和边缘系统的主要靶点的大脑区域,结果显示5-羟色胺显着增加(2倍),但代谢产物不是5-羟基吲哚乙酸。这项研究朝着建立MALDI-MSI作为完全定量验证的方法迈出了实质性的一步。



























 京公网安备 11010802027423号
京公网安备 11010802027423号