当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aqueous Uranium Speciation on U/Ca in Foraminiferal Calcite: The Importance of Minor Species—UO2(CO3)22–
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-10-22 , DOI: 10.1021/acsearthspacechem.0c00211 Xinming Chen 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-10-22 , DOI: 10.1021/acsearthspacechem.0c00211 Xinming Chen 1
Affiliation
|
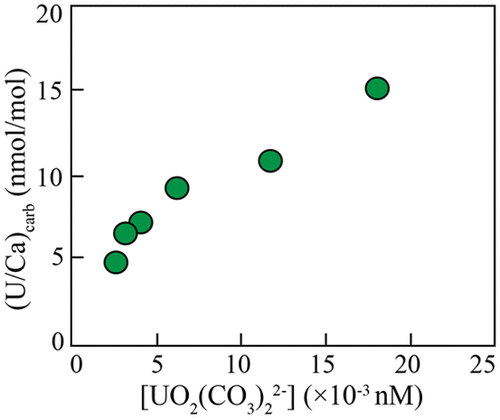
The decrease of uranium to calcium ratios (U/Ca) in biogenic CaCO3 with increasing pH and/or [CO32–] is being explored as a proxy to reconstruct past seawater carbonate chemistry. This inverse relationship was interpreted to reflect the changes in the abundances of dissolved U(VI) species that were incorporated into CaCO3 with pH and/or [CO32–]. Aqueous U(VI) speciation calculations in all published work on U/Ca in CaCO3 only considered UO2-CO3 and UO2-OH complexes. The recent discovery that Ca/Mg-UO2-CO3 complexes are the dominant U(VI) species in seawater rather than UO2-CO3 complexes calls this interpretation into question. Considering the Ca/Mg-UO2-CO3 complexes, I revisited published data of foraminifera culturing experiments to examine the effects of U(VI) speciation on U/Ca in these calcite precipitates. My results reveal that concentrations of Ca/Mg-UO2-CO3 complexes and UO2(CO3)34– remain statistically invariant within uncertainty when the pH and [CO32–] increase, whereas the minor U(VI) species UO2(CO3)22– decreases by 10-fold. The decrease of [UO2(CO3)22–] with increasing pH and [CO32–] best predicts the decrease of U/Ca in foraminifera, suggesting that UO2(CO3)22– is most likely the U(VI) species incorporated into calcite. The incorporation of the minor U(VI) species UO2(CO3)22– into calcite provides an alternative interpretation of significantly lower U concentration in calcite relative to aragonite, if the more abundant UO2(CO3)34– is incorporated into aragonite. Theoretical aqueous U(VI) speciation further demonstrates that [UO2(CO3)22–] is very sensitive to changes in pH, [CO32–], and [Ca2+], suggesting that U/Ca is a good proxy for seawater pH and [CO32–] within ∼400 kyr when both U and Ca concentrations remain constant.
中文翻译:

有孔虫方解石中U / Ca上的铀水溶液形态:次要物种UU 2(CO 3)2 2–的重要性
人们正在探索随着pH和/或[CO 3 2- ]的增加,生物型CaCO 3中铀钙比(U / Ca)的降低,以替代过去的海水碳酸盐化学。这种反比关系被解释为反映了溶解的U(VI)物种的丰度变化,这些物种在pH和/或[CO 3 2– ]下并入CaCO 3中。在CaCO 3中有关U / Ca的所有已发表工作中,U(VI)水溶液的形态计算仅考虑UO 2 -CO 3和UO 2 -OH配合物。Ca / Mg-UO 2 -CO 3的最新发现络合物是海水中主要的U(VI)物种,而不是UO 2 -CO 3络合物使这种解释成为疑问。考虑到Ca / Mg-UO 2 -CO 3配合物,我重新查看了有孔虫培养实验的公开数据,以研究U(VI)形态对这些方解石沉淀物中U / Ca的影响。我的结果表明,当pH和[CO 3 2- ]增加时,Ca / Mg-UO 2 -CO 3复合物和UO 2(CO 3)3 4–的浓度在不确定性内保持统计不变,而次要的U(VI)物种UO 2(CO 3)2 2–减少10倍。随着pH值的升高,[UO 2(CO 3)2 2– ]的降低和[CO 3 2– ]的最佳预测是有孔虫中U / Ca的降低,表明UO 2(CO 3)2 2–最可能是有孔虫。U(VI)物种合并到方解石中。次要U(Ⅵ)物种UO掺入2(CO 3)2 2-成方解石提供的显著底部U浓度相对于文石方解石,可替换的解释,如果更丰富的UO 2(CO 3)3 4–被掺入文石中。U(VI)水溶液的理论形态学进一步证明了[UO 2(CO 3)2 2– ]对pH,[CO 3 2– ]和[Ca 2+ ]的变化非常敏感,表明U / Ca是一种当U和Ca浓度保持恒定时,海水pH和[CO 3 2- ]的良好替代物在约400 kyr内。
更新日期:2020-11-19
中文翻译:

有孔虫方解石中U / Ca上的铀水溶液形态:次要物种UU 2(CO 3)2 2–的重要性
人们正在探索随着pH和/或[CO 3 2- ]的增加,生物型CaCO 3中铀钙比(U / Ca)的降低,以替代过去的海水碳酸盐化学。这种反比关系被解释为反映了溶解的U(VI)物种的丰度变化,这些物种在pH和/或[CO 3 2– ]下并入CaCO 3中。在CaCO 3中有关U / Ca的所有已发表工作中,U(VI)水溶液的形态计算仅考虑UO 2 -CO 3和UO 2 -OH配合物。Ca / Mg-UO 2 -CO 3的最新发现络合物是海水中主要的U(VI)物种,而不是UO 2 -CO 3络合物使这种解释成为疑问。考虑到Ca / Mg-UO 2 -CO 3配合物,我重新查看了有孔虫培养实验的公开数据,以研究U(VI)形态对这些方解石沉淀物中U / Ca的影响。我的结果表明,当pH和[CO 3 2- ]增加时,Ca / Mg-UO 2 -CO 3复合物和UO 2(CO 3)3 4–的浓度在不确定性内保持统计不变,而次要的U(VI)物种UO 2(CO 3)2 2–减少10倍。随着pH值的升高,[UO 2(CO 3)2 2– ]的降低和[CO 3 2– ]的最佳预测是有孔虫中U / Ca的降低,表明UO 2(CO 3)2 2–最可能是有孔虫。U(VI)物种合并到方解石中。次要U(Ⅵ)物种UO掺入2(CO 3)2 2-成方解石提供的显著底部U浓度相对于文石方解石,可替换的解释,如果更丰富的UO 2(CO 3)3 4–被掺入文石中。U(VI)水溶液的理论形态学进一步证明了[UO 2(CO 3)2 2– ]对pH,[CO 3 2– ]和[Ca 2+ ]的变化非常敏感,表明U / Ca是一种当U和Ca浓度保持恒定时,海水pH和[CO 3 2- ]的良好替代物在约400 kyr内。


























 京公网安备 11010802027423号
京公网安备 11010802027423号