当前位置:
X-MOL 学术
›
Bioeng. Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of an alkyl spacer on the morphology and internalization of MUC1 aptamer‐naphthalimide amphiphiles for targeting and imaging triple negative breast cancer cells
Bioengineering & Translational Medicine ( IF 7.4 ) Pub Date : 2020-10-21 , DOI: 10.1002/btm2.10194 Huihui Kuang 1 , Zachary Schneiderman 1, 2 , Ahmed M Shabana 1, 3 , Gabriella C Russo 1, 2 , Jun Guo 1 , Denis Wirtz 1, 2 , Efrosini Kokkoli 1, 2
Bioengineering & Translational Medicine ( IF 7.4 ) Pub Date : 2020-10-21 , DOI: 10.1002/btm2.10194 Huihui Kuang 1 , Zachary Schneiderman 1, 2 , Ahmed M Shabana 1, 3 , Gabriella C Russo 1, 2 , Jun Guo 1 , Denis Wirtz 1, 2 , Efrosini Kokkoli 1, 2
Affiliation
|
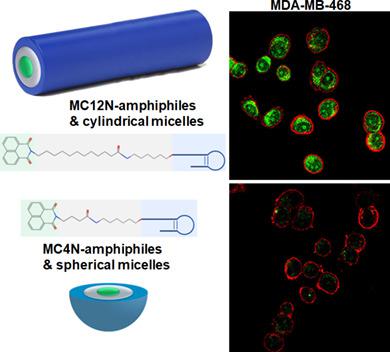
Despite decades of research, there are few targeted treatment options available for triple negative breast cancer (TNBC), leaving chemotherapy, and radiation treatment regimes with poor response and high toxicity. Herein aptamer‐amphiphiles were synthesized which selectively bind to the mucin‐1 (MUC1) glycoprotein that is overexpressed in TNBC cells. These amphiphiles have a fluorescent tail (1,8‐naphthalimide or 4‐nitro‐1,8‐naphthalimide) which enables self‐assembly of the amphiphiles and allows for easy visualization without the requirement for further conjugation of a fluorophore. Interestingly, the length of the alkyl spacer (C4 or C12) between the aptamer and tail was shown to influence the morphology of the self‐assembled structure, and thus its ability to internalize into the TNBC cells. While both the MUC1 aptamer‐C4‐napthalimide spherical micelles and the MUC1 aptamer‐C12‐napthalimide long cylindrical micelles showed internalization into MDA‐MB‐468 TNBC cells but not the noncancerous MCF‐10A breast cells, the cylindrical micelles showed greatly enhanced internalization into the MDA‐MB‐468 cells. Similar patterns of enhanced binding and internalization were observed between the MUC1 aptamer‐C12‐napthalimide cylindrical micelles and SUM159 and MDA‐MB‐231 TNBC cells. The MUC1 aptamer cylindrical micelles were not toxic to the cells, and when used to deliver doxorubicin to the TNBC cells, were shown to be as cytotoxic as free doxorubicin. Moreover, a pharmacokinetic study in mice showed a prolonged systemic circulation time of the MUC1 aptamer cylindrical micelles. There was a 4.6‐fold increase in the elimination half‐life of the aptamer cylindrical micelles, and their clearance decreased 10‐fold compared to the MUC1 aptamer spherical micelles. Thus, the MUC1 aptamer‐C12‐napthalimide nanofibers represent a promising vehicle that could be used for easy visualization and targeted delivery of therapeutic loads to TNBC cells.
中文翻译:

烷基间隔物对 MUC1 适配体-萘酰亚胺两亲物的形态和内化的影响,用于靶向和成像三阴性乳腺癌细胞
尽管进行了数十年的研究,但三阴性乳腺癌 (TNBC) 的靶向治疗方案很少,因此化疗和放射治疗方案的反应较差且毒性较大。在此合成了适体-两亲物,它们选择性地结合在 TNBC 细胞中过表达的粘蛋白-1 (MUC1) 糖蛋白。这些两亲物具有荧光尾(1,8-萘酰亚胺或 4-硝基-1,8-萘酰亚胺),可实现两亲物的自组装,无需进一步结合荧光团即可轻松观察。有趣的是,烷基间隔的长度(C 4或 C 12)在适配体和尾部之间显示出影响自组装结构的形态,从而影响其内化到 TNBC 细胞中的能力。虽然 MUC1 适体-C 4 -萘酰亚胺球形胶束和 MUC1 适体-C 12 -萘酰亚胺长圆柱形胶束均显示内化到 MDA-MB-468 TNBC 细胞中,但未内化到非癌性 MCF-10A 乳腺细胞中,但圆柱形胶束显示出显着增强内化到 MDA-MB-468 细胞中。在 MUC1 aptamer-C 12之间观察到类似的增强结合和内化模式-萘酰亚胺圆柱胶束和 SUM159 和 MDA-MB-231 TNBC 细胞。MUC1 适体圆柱形胶束对细胞没有毒性,并且当用于将阿霉素递送至 TNBC 细胞时,显示出与游离阿霉素一样具有细胞毒性。此外,在小鼠中进行的药代动力学研究表明,MUC1 适体圆柱形胶束的全身循环时间延长。与 MUC1 适体球形胶束相比,适体圆柱胶束的消除半衰期增加了 4.6 倍,清除率降低了 10 倍。因此,MUC1 适体-C 12 -萘酰亚胺纳米纤维代表了一种有前途的载体,可用于轻松可视化和将治疗负荷靶向递送至 TNBC 细胞。
更新日期:2020-10-21
中文翻译:

烷基间隔物对 MUC1 适配体-萘酰亚胺两亲物的形态和内化的影响,用于靶向和成像三阴性乳腺癌细胞
尽管进行了数十年的研究,但三阴性乳腺癌 (TNBC) 的靶向治疗方案很少,因此化疗和放射治疗方案的反应较差且毒性较大。在此合成了适体-两亲物,它们选择性地结合在 TNBC 细胞中过表达的粘蛋白-1 (MUC1) 糖蛋白。这些两亲物具有荧光尾(1,8-萘酰亚胺或 4-硝基-1,8-萘酰亚胺),可实现两亲物的自组装,无需进一步结合荧光团即可轻松观察。有趣的是,烷基间隔的长度(C 4或 C 12)在适配体和尾部之间显示出影响自组装结构的形态,从而影响其内化到 TNBC 细胞中的能力。虽然 MUC1 适体-C 4 -萘酰亚胺球形胶束和 MUC1 适体-C 12 -萘酰亚胺长圆柱形胶束均显示内化到 MDA-MB-468 TNBC 细胞中,但未内化到非癌性 MCF-10A 乳腺细胞中,但圆柱形胶束显示出显着增强内化到 MDA-MB-468 细胞中。在 MUC1 aptamer-C 12之间观察到类似的增强结合和内化模式-萘酰亚胺圆柱胶束和 SUM159 和 MDA-MB-231 TNBC 细胞。MUC1 适体圆柱形胶束对细胞没有毒性,并且当用于将阿霉素递送至 TNBC 细胞时,显示出与游离阿霉素一样具有细胞毒性。此外,在小鼠中进行的药代动力学研究表明,MUC1 适体圆柱形胶束的全身循环时间延长。与 MUC1 适体球形胶束相比,适体圆柱胶束的消除半衰期增加了 4.6 倍,清除率降低了 10 倍。因此,MUC1 适体-C 12 -萘酰亚胺纳米纤维代表了一种有前途的载体,可用于轻松可视化和将治疗负荷靶向递送至 TNBC 细胞。


























 京公网安备 11010802027423号
京公网安备 11010802027423号