当前位置:
X-MOL 学术
›
Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Advances in Lentivirus Purification
Biotechnology Journal ( IF 4.7 ) Pub Date : 2020-10-22 , DOI: 10.1002/biot.202000019 Ana Sofia Moreira 1, 2 , David Guia Cavaco 1, 2 , Tiago Q. Faria 1, 2 , Paula M. Alves 1, 2 , Manuel J. T. Carrondo 1 , Cristina Peixoto 1
Biotechnology Journal ( IF 4.7 ) Pub Date : 2020-10-22 , DOI: 10.1002/biot.202000019 Ana Sofia Moreira 1, 2 , David Guia Cavaco 1, 2 , Tiago Q. Faria 1, 2 , Paula M. Alves 1, 2 , Manuel J. T. Carrondo 1 , Cristina Peixoto 1
Affiliation
|
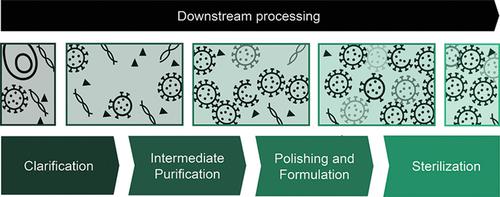
Lentiviral vectors (LVs) have been increasingly used as a tool for gene and cell therapies since they can stably integrate the genome in dividing and nondividing cells. LV production and purification processes have evolved substantially over the last decades. However, the increasing demands for higher quantities with more restrictive purity requirements are stimulating the development of novel materials and strategies to supply the market with LV in a cost‐effective manner. A detailed review of each downstream process unit operation is performed, limitations, strengths, and potential outcomes being covered. Currently, the majority of large‐scale LV manufacturing processes are still based on adherent cell culture, although it is known that the industry is migrating fast to suspension cultures. Regarding the purification strategy, it consists of batch chromatography and membrane technology. Nevertheless, new solutions are being created to improve the current production schemes and expand its clinical use.
中文翻译:

慢病毒纯化的进展
慢病毒载体(LVs)已越来越多地用作基因和细胞疗法的工具,因为它们可以将基因组稳定地整合到分裂和非分裂细胞中。在过去的几十年中,LV的生产和纯化工艺已经有了实质性的发展。但是,对更高数量的需求以及对纯度的要求越来越严格,这刺激了新型材料和策略的发展,从而以经济高效的方式向市场供应LV。对每个下游处理单元操作进行了详细的审查,包括了局限性,优势和潜在成果。当前,尽管已知该行业正在迅速向悬浮培养迁移,但大多数大型LV制造过程仍基于贴壁细胞培养。关于净化策略,它由间歇色谱和膜技术组成。然而,正在开发新的解决方案以改善当前的生产方案并扩大其临床用途。
更新日期:2020-10-22
中文翻译:

慢病毒纯化的进展
慢病毒载体(LVs)已越来越多地用作基因和细胞疗法的工具,因为它们可以将基因组稳定地整合到分裂和非分裂细胞中。在过去的几十年中,LV的生产和纯化工艺已经有了实质性的发展。但是,对更高数量的需求以及对纯度的要求越来越严格,这刺激了新型材料和策略的发展,从而以经济高效的方式向市场供应LV。对每个下游处理单元操作进行了详细的审查,包括了局限性,优势和潜在成果。当前,尽管已知该行业正在迅速向悬浮培养迁移,但大多数大型LV制造过程仍基于贴壁细胞培养。关于净化策略,它由间歇色谱和膜技术组成。然而,正在开发新的解决方案以改善当前的生产方案并扩大其临床用途。



























 京公网安备 11010802027423号
京公网安备 11010802027423号