当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
PASS-DIA: A Data-Independent Acquisition Approach for Discovery Studies
Analytical Chemistry ( IF 7.4 ) Pub Date : 2020-10-20 , DOI: 10.1021/acs.analchem.0c02513 Dong-Gi Mun 1 , Santosh Renuse 1, 2 , Mayank Saraswat 1, 3, 4, 5 , Anil Madugundu 1, 2, 3, 4, 5 , Savita Udainiya 1, 2, 3 , Hokeun Kim 6 , Sung-Kyu Robin Park 7 , Hui Zhao 1 , Raja Sekhar Nirujogi 8 , Chan Hyun Na 9, 10 , Nagarajan Kannan 1 , John R. Yates 7 , Sang-Won Lee 6 , Akhilesh Pandey 1, 2, 3, 4, 5
Analytical Chemistry ( IF 7.4 ) Pub Date : 2020-10-20 , DOI: 10.1021/acs.analchem.0c02513 Dong-Gi Mun 1 , Santosh Renuse 1, 2 , Mayank Saraswat 1, 3, 4, 5 , Anil Madugundu 1, 2, 3, 4, 5 , Savita Udainiya 1, 2, 3 , Hokeun Kim 6 , Sung-Kyu Robin Park 7 , Hui Zhao 1 , Raja Sekhar Nirujogi 8 , Chan Hyun Na 9, 10 , Nagarajan Kannan 1 , John R. Yates 7 , Sang-Won Lee 6 , Akhilesh Pandey 1, 2, 3, 4, 5
Affiliation
|
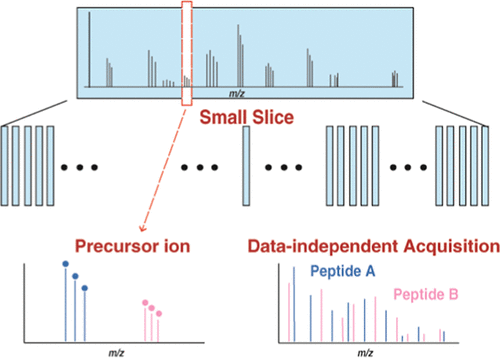
A data-independent acquisition (DIA) approach is being increasingly adopted as a promising strategy for identification and quantitation of proteomes. As most DIA data sets are acquired with wide isolation windows, highly complex MS/MS spectra are generated, which negatively impacts obtaining peptide information through classical protein database searches. Therefore, the analysis of DIA data mainly relies on the evidence of the existence of peptides from prebuilt spectral libraries. Consequently, one major weakness of this method is that it does not account for peptides that are not included in the spectral library, precluding the use of DIA for discovery studies. Here, we present a strategy termed Precursor ion And Small Slice-DIA (PASS-DIA) in which MS/MS spectra are acquired with small isolation windows (slices) and MS/MS spectra are interpreted with accurately determined precursor ion masses. This method enables the direct application of conventional spectrum-centric analysis pipelines for peptide identification and precursor ion-based quantitation. The performance of PASS-DIA was observed to be superior to both data-dependent acquisition (DDA) and conventional DIA experiments with 69 and 48% additional protein identifications, respectively. Application of PASS-DIA for the analysis of post-translationally modified peptides again highlighted its superior performance in characterizing phosphopeptides (77% more), N-terminal acetylated peptides (56% more), and N-glycopeptides (83% more) as compared to DDA alone. Finally, the use of PASS-DIA to characterize a rare proteome of human fallopian tube organoids enabled 34% additional protein identifications than DDA alone and revealed biologically relevant pathways including low abundance proteins. Overall, PASS-DIA is a novel DIA approach for use as a discovery tool that outperforms both conventional DDA and DIA experiments to provide additional protein information. We believe that the PASS-DIA method is an important strategy for discovery-type studies when deeper proteome characterization is required.
中文翻译:

PASS-DIA:发现研究的独立于数据的获取方法
越来越多地采用数据独立获取(DIA)方法作为鉴定和定量蛋白质组学的有前途的策略。由于大多数DIA数据集都是通过宽隔离窗口获取的,因此生成了高度复杂的MS / MS谱图,这不利于通过经典蛋白质数据库搜索获得肽段信息。因此,DIA数据的分析主要依赖于预建光谱库中存在肽的证据。因此,该方法的一个主要缺点是它不能解决光谱库中未包括的肽问题,因此不能将DIA用于发现研究。这里,我们提出了一种称为“前体离子和小切片DIA(PASS-DIA)”的策略,其中MS / MS谱图通过小的隔离窗口(切片)获取,MS / MS谱图以准确确定的前体离子质量进行解释。该方法可将常规的以谱为中心的分析管线直接用于肽鉴定和基于前体离子的定量。观察到PASS-DIA的性能优于数据依赖采集(DDA)和常规DIA实验,分别具有69%和48%的额外蛋白质鉴定。PASS-DIA在分析翻译后修饰的肽中的应用再次凸显了其在表征磷酸肽(增加77%),N末端乙酰化肽(增加56%)和N-糖肽(增加83%)方面的优异性能。单独使用DDA。最后,使用PASS-DIA表征人类输卵管类器官的稀有蛋白质组比单独使用DDA可以进行34%的额外蛋白质鉴定,并揭示了包括低丰度蛋白质在内的生物学相关途径。总体而言,PASS-DIA是一种新颖的DIA方法,可用作发现工具,其性能优于常规DDA和DIA实验,可提供更多的蛋白质信息。我们认为,当需要更深入的蛋白质组表征时,PASS-DIA方法是发现型研究的重要策略。PASS-DIA是一种新颖的DIA方法,可用作发现工具,其性能优于常规DDA和DIA实验,可提供更多的蛋白质信息。我们认为,当需要更深入的蛋白质组表征时,PASS-DIA方法是发现型研究的重要策略。PASS-DIA是一种新颖的DIA方法,可用作发现工具,其性能优于常规DDA和DIA实验,可提供更多的蛋白质信息。我们认为,当需要更深入的蛋白质组表征时,PASS-DIA方法是发现型研究的重要策略。
更新日期:2020-11-03
中文翻译:

PASS-DIA:发现研究的独立于数据的获取方法
越来越多地采用数据独立获取(DIA)方法作为鉴定和定量蛋白质组学的有前途的策略。由于大多数DIA数据集都是通过宽隔离窗口获取的,因此生成了高度复杂的MS / MS谱图,这不利于通过经典蛋白质数据库搜索获得肽段信息。因此,DIA数据的分析主要依赖于预建光谱库中存在肽的证据。因此,该方法的一个主要缺点是它不能解决光谱库中未包括的肽问题,因此不能将DIA用于发现研究。这里,我们提出了一种称为“前体离子和小切片DIA(PASS-DIA)”的策略,其中MS / MS谱图通过小的隔离窗口(切片)获取,MS / MS谱图以准确确定的前体离子质量进行解释。该方法可将常规的以谱为中心的分析管线直接用于肽鉴定和基于前体离子的定量。观察到PASS-DIA的性能优于数据依赖采集(DDA)和常规DIA实验,分别具有69%和48%的额外蛋白质鉴定。PASS-DIA在分析翻译后修饰的肽中的应用再次凸显了其在表征磷酸肽(增加77%),N末端乙酰化肽(增加56%)和N-糖肽(增加83%)方面的优异性能。单独使用DDA。最后,使用PASS-DIA表征人类输卵管类器官的稀有蛋白质组比单独使用DDA可以进行34%的额外蛋白质鉴定,并揭示了包括低丰度蛋白质在内的生物学相关途径。总体而言,PASS-DIA是一种新颖的DIA方法,可用作发现工具,其性能优于常规DDA和DIA实验,可提供更多的蛋白质信息。我们认为,当需要更深入的蛋白质组表征时,PASS-DIA方法是发现型研究的重要策略。PASS-DIA是一种新颖的DIA方法,可用作发现工具,其性能优于常规DDA和DIA实验,可提供更多的蛋白质信息。我们认为,当需要更深入的蛋白质组表征时,PASS-DIA方法是发现型研究的重要策略。PASS-DIA是一种新颖的DIA方法,可用作发现工具,其性能优于常规DDA和DIA实验,可提供更多的蛋白质信息。我们认为,当需要更深入的蛋白质组表征时,PASS-DIA方法是发现型研究的重要策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号