当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbenaporphyrins: No Longer Missing Ligands in N‐Heterocyclic Carbene Chemistry
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-10-20 , DOI: 10.1002/anie.202013434 Theo Maulbetsch 1 , Doris Kunz 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-10-20 , DOI: 10.1002/anie.202013434 Theo Maulbetsch 1 , Doris Kunz 1
Affiliation
|
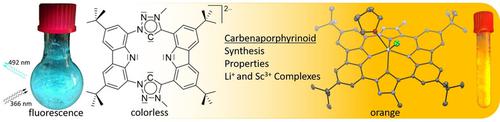
The synthesis of an NHC‐containing porphyrinoid ligand is presented. The formally antiaromatic 20 πe− macrocyclic framework can be obtained via a 1,3‐dipolar cycloaddition (“click‐reaction”) to form two triazole moieties which were alkylated to the respective triazolium macrocycle. Deprotonation of the ligand precursor with lithium bases to the respective dilithio carbenaporphyrin complex and transmetallation to scandium lead to complexes that exhibit orange fluorescence. Optical property combined with TD‐DFT studies verify an aromatic character for each heterocyclic moiety rather than an antiaromatic macrocycle in the ligand precursor as well as in the complexes. While the geometric features of the carbenaporphyrin ligand strongly resemble those of porphyrin, DFT calculations reveal a stronger electron‐donating ability of the new ligand.
中文翻译:

碳卟啉:N-杂环卡宾化学中不再缺少配体
介绍了含 NHC 的卟啉配体的合成。形式上的反芳香族 20 πe -大环骨架可以通过 1,3-偶极环加成(“点击反应”)获得,形成两个三唑部分,这些部分被烷基化为各自的三唑大环。配体前体与锂碱的去质子化形成相应的二锂碳卟啉络合物,并通过金属转移形成钪,导致络合物表现出橙色荧光。光学性质与 TD-DFT 研究相结合,验证了配体前体以及配合物中每个杂环部分的芳香特征,而不是反芳香大环。虽然碳卟啉配体的几何特征与卟啉非常相似,但 DFT 计算表明新配体具有更强的给电子能力。
更新日期:2020-10-20
中文翻译:

碳卟啉:N-杂环卡宾化学中不再缺少配体
介绍了含 NHC 的卟啉配体的合成。形式上的反芳香族 20 πe -大环骨架可以通过 1,3-偶极环加成(“点击反应”)获得,形成两个三唑部分,这些部分被烷基化为各自的三唑大环。配体前体与锂碱的去质子化形成相应的二锂碳卟啉络合物,并通过金属转移形成钪,导致络合物表现出橙色荧光。光学性质与 TD-DFT 研究相结合,验证了配体前体以及配合物中每个杂环部分的芳香特征,而不是反芳香大环。虽然碳卟啉配体的几何特征与卟啉非常相似,但 DFT 计算表明新配体具有更强的给电子能力。

























 京公网安备 11010802027423号
京公网安备 11010802027423号