当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical 1,2‐Diarylation of Alkenes Enabled by Direct Dual C–H Functionalizations of Electron‐Rich Aromatic Hydrocarbons
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-10-20 , DOI: 10.1002/anie.202011657 Jing‐Hao Qin 1, 2 , Mu‐Jia Luo 1, 2 , De‐Lie An 1, 2 , Jin‐Heng Li 1, 2, 3, 4
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-10-20 , DOI: 10.1002/anie.202011657 Jing‐Hao Qin 1, 2 , Mu‐Jia Luo 1, 2 , De‐Lie An 1, 2 , Jin‐Heng Li 1, 2, 3, 4
Affiliation
|
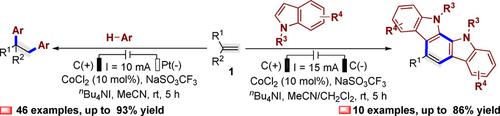
A cobalt‐promoted electrochemical 1,2‐diarylation of alkenes with electron‐rich aromatic hydrocarbons via direct dual C−H functionalizations is described, which employs a radical relay strategy to produce polyaryl‐functionalized alkanes. Simply by using graphite rod cathode instead of platinum plate cathode, chemoselectivity of this radical relay strategy is shifted to the dehydrogenative [2+2+2] cycloaddition via 1,2‐diarylation, annulation, and dehydrogenation cascades leading to complex 11,12‐dihydroindolo[2,3‐a]carbazoles. Mechanistical studies indicate that a key step for the radical relay processes is transformations of the aromatic hydrocarbons to the aryl sp2‐hybridized carbon‐centered radicals via deprotonation of the corresponding aryl radical cation intermediates with bases.
中文翻译:

富含电子的芳烃的直接双C–H官能化作用使烯烃的1,2-二芳基化
描述了通过直接双CH功能将钴与富电子芳烃进行烯烃促进的电化学1,2-二芳基化反应,该方法采用自由基中继策略来生产聚芳基官能化的烷烃。只需使用石墨棒阴极而不是铂板阴极,这种自由基中继策略的化学选择性就可以通过1,2-二芳基化,环化和脱氢级联反应转变为脱氢[2 + 2 + 2]环加成反应,从而形成复杂的11,12-二氢吲哚[2,3- a ]咔唑。机理研究表明,自由基中继过程的关键步骤是将芳烃转化为芳基sp 2通过使相应的芳基自由基阳离子中间体与碱去质子化,使碳中心自由基杂交。
更新日期:2020-10-20
中文翻译:

富含电子的芳烃的直接双C–H官能化作用使烯烃的1,2-二芳基化
描述了通过直接双CH功能将钴与富电子芳烃进行烯烃促进的电化学1,2-二芳基化反应,该方法采用自由基中继策略来生产聚芳基官能化的烷烃。只需使用石墨棒阴极而不是铂板阴极,这种自由基中继策略的化学选择性就可以通过1,2-二芳基化,环化和脱氢级联反应转变为脱氢[2 + 2 + 2]环加成反应,从而形成复杂的11,12-二氢吲哚[2,3- a ]咔唑。机理研究表明,自由基中继过程的关键步骤是将芳烃转化为芳基sp 2通过使相应的芳基自由基阳离子中间体与碱去质子化,使碳中心自由基杂交。



























 京公网安备 11010802027423号
京公网安备 11010802027423号