当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Artificial ER-Derived Vesicles as Synthetic Organelles for in Vivo Compartmentalization of Biochemical Pathways
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-10-19 , DOI: 10.1021/acssynbio.0c00241 Mara Reifenrath 1 , Mislav Oreb 1 , Eckhard Boles 1 , Joanna Tripp 1
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-10-19 , DOI: 10.1021/acssynbio.0c00241 Mara Reifenrath 1 , Mislav Oreb 1 , Eckhard Boles 1 , Joanna Tripp 1
Affiliation
|
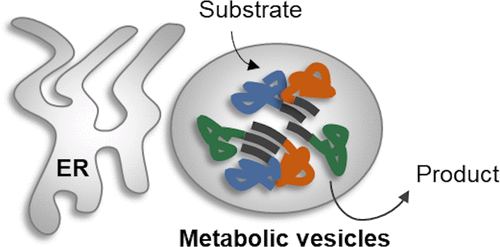
Compartmentalization in membrane-surrounded organelles has the potential to overcome obstacles associated with the engineering of metabolic pathways, such as unwanted side reactions, accumulation of toxic intermediates, drain of intermediates out of the cell, and long diffusion distances. Strategies utilizing natural organelles suffer from the presence of endogenous pathways. In our approach, we make use of endoplasmic reticulum-derived vesicles loaded with enzymes of a metabolic pathway (“metabolic vesicles”). They are generated by fusion of synthetic peptides containing the N-terminal proline-rich and self-assembling region of the maize storage protein gamma-Zein (“Zera”) to the pathway enzymes. We have applied a strategy to integrate three enzymes of a cis,cis-muconic acid production pathway into those vesicles in yeast. Using fluorescence microscopy and cell fractionation techniques, we have proven the formation of metabolic vesicles and the incorporation of enzymes. Activities of the enzymes and functionality of the compartmentalized pathway were demonstrated in fermentation experiments.
中文翻译:

人工 ER 衍生的囊泡作为合成细胞器用于生物化学途径的体内区室化
膜包围细胞器中的区室化有可能克服与代谢途径工程相关的障碍,例如不需要的副反应、有毒中间体的积累、中间体从细胞中排出以及长扩散距离。利用天然细胞器的策略受到内源性途径的影响。在我们的方法中,我们利用装载有代谢途径酶的内质网衍生囊泡(“代谢囊泡”)。它们是通过含有玉米储存蛋白 γ-玉米醇溶蛋白(“Zera”)的 N 端富含脯氨酸和自组装区域的合成肽与途径酶融合而产生的。我们应用了一种策略来整合顺式,顺式三种酶-粘康酸生产途径进入酵母中的那些囊泡。使用荧光显微镜和细胞分级技术,我们已经证明了代谢囊泡的形成和酶的掺入。在发酵实验中证明了酶的活性和区室化途径的功能。
更新日期:2020-11-21
中文翻译:

人工 ER 衍生的囊泡作为合成细胞器用于生物化学途径的体内区室化
膜包围细胞器中的区室化有可能克服与代谢途径工程相关的障碍,例如不需要的副反应、有毒中间体的积累、中间体从细胞中排出以及长扩散距离。利用天然细胞器的策略受到内源性途径的影响。在我们的方法中,我们利用装载有代谢途径酶的内质网衍生囊泡(“代谢囊泡”)。它们是通过含有玉米储存蛋白 γ-玉米醇溶蛋白(“Zera”)的 N 端富含脯氨酸和自组装区域的合成肽与途径酶融合而产生的。我们应用了一种策略来整合顺式,顺式三种酶-粘康酸生产途径进入酵母中的那些囊泡。使用荧光显微镜和细胞分级技术,我们已经证明了代谢囊泡的形成和酶的掺入。在发酵实验中证明了酶的活性和区室化途径的功能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号