当前位置:
X-MOL 学术
›
Macromol. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Initiation and Termination in Styrene Free‐Radical Polymerization Initiated by Redox Initiation
Macromolecular Chemistry and Physics ( IF 2.5 ) Pub Date : 2020-10-19 , DOI: 10.1002/macp.202000277 Hongfei Han 1 , Jianhan Li 1 , Wenyan Huang 1 , Qimin Jiang 1 , Li Jiang 1 , Xiaoqiang Xue 1 , Hongjun Yang 1 , Bibiao Jiang 1, 2
Macromolecular Chemistry and Physics ( IF 2.5 ) Pub Date : 2020-10-19 , DOI: 10.1002/macp.202000277 Hongfei Han 1 , Jianhan Li 1 , Wenyan Huang 1 , Qimin Jiang 1 , Li Jiang 1 , Xiaoqiang Xue 1 , Hongjun Yang 1 , Bibiao Jiang 1, 2
Affiliation
|
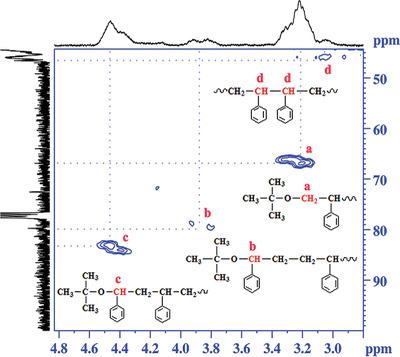
Hydrogen peroxide and hydroperoxides with substituents of different size are combined with ferrous sulfate to form redox initiation systems, which are used to initiate the polymerization of styrene in emulsion. Gas chromatography and size‐exclusion chromatography are used to measure the monomer conversion and the molecular weight of the polystyrene. Nuclear magnetic resonance is used to identify the characteristic structures, quantitative information is used to understand the polymerization. The results suggest that the initiation of the primary radicals directly depend on the size of the substitute, hydroxyl radical shows almost no selectivity between head‐addition and tail‐addition during initiation (Fhi = 47.4%). But for primary radicals with big substitute groups, for example, t‐butyl and cumyl hydroperoxides, tail‐addition takes advantages over head‐addition during initiation (Fhi ≈80%). As for the termination mechanism, it mainly depends on the solubility of the peroxide in water, the interfacial area of the particle as well as the diffusion rate of the primary radical in aqueous phase. Primary termination dominates in the polymerization initiated by hydroperoxide with poor solubility in water, for example, Fpt = 75–80% for t‐butyl and cumyl hydroperoxides. But Fpt is only 18.3% in the polymerization initiated by hydrogen peroxide (H2O2), indicating coupling termination predominately occurred because H2O2 has excellent solubility in water.
中文翻译:

由氧化还原引发引发的苯乙烯自由基聚合中的引发和终止
将具有不同尺寸取代基的过氧化氢和氢过氧化物与硫酸亚铁结合形成氧化还原引发体系,该体系用于引发乳液中苯乙烯的聚合。气相色谱法和尺寸排阻色谱法用于测量单体转化率和聚苯乙烯的分子量。核磁共振用于识别特征结构,定量信息用于理解聚合。结果表明,伯自由基的引发直接取决于取代基的大小,羟自由基在引发期间在头加和尾加之间几乎没有选择性(F hi = 47.4%)。但是,对于具有大量替代基团的主要部首,例如,t丁基枯基氢过氧化物,尾加成发起期间接管头加成优点(˚F喜 ≈80%)。至于终止机理,它主要取决于过氧化物在水中的溶解度,颗粒的界面面积以及伯自由基在水相中的扩散速率。初级终止反应在氢过氧化物引发的聚合反应中占主导地位,而氢过氧化物在水中的溶解性较差,例如叔丁基和枯基氢过氧化物的F pt = 75-80%。但是在过氧化氢(H 2 O 2)引发的聚合反应中,F pt仅为18.3%,这主要是由于H 2导致了偶合终止。O 2在水中具有极好的溶解性。
更新日期:2020-11-19
中文翻译:

由氧化还原引发引发的苯乙烯自由基聚合中的引发和终止
将具有不同尺寸取代基的过氧化氢和氢过氧化物与硫酸亚铁结合形成氧化还原引发体系,该体系用于引发乳液中苯乙烯的聚合。气相色谱法和尺寸排阻色谱法用于测量单体转化率和聚苯乙烯的分子量。核磁共振用于识别特征结构,定量信息用于理解聚合。结果表明,伯自由基的引发直接取决于取代基的大小,羟自由基在引发期间在头加和尾加之间几乎没有选择性(F hi = 47.4%)。但是,对于具有大量替代基团的主要部首,例如,t丁基枯基氢过氧化物,尾加成发起期间接管头加成优点(˚F喜 ≈80%)。至于终止机理,它主要取决于过氧化物在水中的溶解度,颗粒的界面面积以及伯自由基在水相中的扩散速率。初级终止反应在氢过氧化物引发的聚合反应中占主导地位,而氢过氧化物在水中的溶解性较差,例如叔丁基和枯基氢过氧化物的F pt = 75-80%。但是在过氧化氢(H 2 O 2)引发的聚合反应中,F pt仅为18.3%,这主要是由于H 2导致了偶合终止。O 2在水中具有极好的溶解性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号