当前位置:
X-MOL 学术
›
Cytom. Part A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stabilization of Human Whole Blood Samples for Multicenter and Retrospective Immunophenotyping Studies
Cytometry Part A ( IF 3.7 ) Pub Date : 2020-10-18 , DOI: 10.1002/cyto.a.24241 Paulina Rybakowska 1 , Catalina Burbano 2 , Sofie Van Gassen 3, 4 , Nieves Varela 1 , Rocío Aguilar-Quesada 5 , Yvan Saeys 3, 4 , Marta E Alarcón-Riquelme 1, 6 , Concepción Marañón 1
Cytometry Part A ( IF 3.7 ) Pub Date : 2020-10-18 , DOI: 10.1002/cyto.a.24241 Paulina Rybakowska 1 , Catalina Burbano 2 , Sofie Van Gassen 3, 4 , Nieves Varela 1 , Rocío Aguilar-Quesada 5 , Yvan Saeys 3, 4 , Marta E Alarcón-Riquelme 1, 6 , Concepción Marañón 1
Affiliation
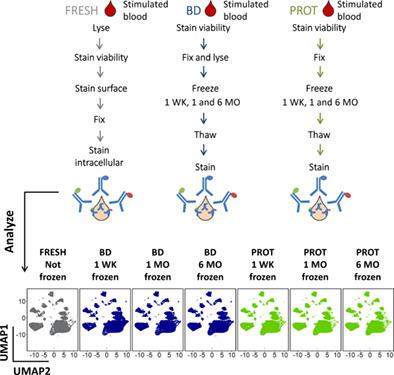
|
Whole blood is often collected for large‐scale immune monitoring studies to track changes in cell frequencies and responses using flow (FC) or mass cytometry (MC). In order to preserve sample composition and phenotype, blood samples should be analyzed within 24 h after bleeding, restricting the recruitment, analysis protocols, as well as biobanking. Herein, we have evaluated two whole blood preservation protocols that allow rapid sample processing and long‐term stability. Two fixation buffers were used, Phosphoflow Fix and Lyse (BD) and Proteomic Stabilizer (PROT) to fix and freeze whole blood samples for up to 6 months. After analysis by an 8‐plex panel by FC and a 26‐plex panel by MC, manual gating of circulating leukocyte populations and cytokines was performed. Additionally, we tested the stability of a single sample over a 13‐months period using 45 consecutive aliquots and a 34‐plex panel by MC. We observed high correlation and low bias toward any cell population when comparing fresh and 6 months frozen blood with FC and MC. This correlation was confirmed by hierarchical clustering. Low coefficients of variation (CV) across studied time points indicate good sample preservation for up to 6 months. Cytokine detection stability was confirmed by low CVs, with some differences between fresh and fixed conditions. Thirteen months regular follow‐up of PROT samples showed remarkable sample stability. Whole blood can be preserved for phenotyping and cytokine‐response studies provided the careful selection of a compatible antibody panel. However, possible changes in cell morphology, differences in antibody affinity, and changes in cytokine‐positive cell frequencies when compared to fresh blood should be considered. Our setting constitutes a valuable tool for multicentric and retrospective studies. © 2020 International Society for Advancement of Cytometry
中文翻译:

用于多中心和回顾性免疫表型研究的人全血样品的稳定性
通常收集全血用于大规模免疫监测研究,以使用流式 (FC) 或质谱流式细胞术 (MC) 跟踪细胞频率和反应的变化。为了保留样本组成和表型,应在出血后 24 小时内分析血液样本,限制招募、分析协议以及生物银行。在此,我们评估了两种全血保存方案,可实现快速样品处理和长期稳定性。使用两种固定缓冲液、Phosphoflow Fix and Lyse (BD) 和蛋白质组稳定剂 (PROT) 来固定和冷冻全血样品长达 6 个月。通过 FC 的 8 重面板和 MC 的 26 重面板分析后,对循环白细胞群和细胞因子进行手动门控。此外,我们使用 45 个连续的等分试样和 MC 的 34 重面板测试了单个样品在 13 个月内的稳定性。在将新鲜和 6 个月冷冻血液与 FC 和 MC 进行比较时,我们观察到对任何细胞群的高度相关性和低偏差。这种相关性通过层次聚类得到证实。跨研究时间点的低变异系数 (CV) 表明样品保存良好长达 6 个月。细胞因子检测稳定性通过低 CV 得到证实,新鲜和固定条件之间存在一些差异。PROT 样品的 13 个月定期随访显示出显着的样品稳定性。如果仔细选择兼容的抗体组,可以保存全血用于表型分析和细胞因子反应研究。然而,细胞形态的可能变化、抗体亲和力的差异、应考虑与新鲜血液相比细胞因子阳性细胞频率的变化。我们的环境构成了多中心和回顾性研究的宝贵工具。© 2020 国际细胞计量学促进会
更新日期:2020-10-18
中文翻译:

用于多中心和回顾性免疫表型研究的人全血样品的稳定性
通常收集全血用于大规模免疫监测研究,以使用流式 (FC) 或质谱流式细胞术 (MC) 跟踪细胞频率和反应的变化。为了保留样本组成和表型,应在出血后 24 小时内分析血液样本,限制招募、分析协议以及生物银行。在此,我们评估了两种全血保存方案,可实现快速样品处理和长期稳定性。使用两种固定缓冲液、Phosphoflow Fix and Lyse (BD) 和蛋白质组稳定剂 (PROT) 来固定和冷冻全血样品长达 6 个月。通过 FC 的 8 重面板和 MC 的 26 重面板分析后,对循环白细胞群和细胞因子进行手动门控。此外,我们使用 45 个连续的等分试样和 MC 的 34 重面板测试了单个样品在 13 个月内的稳定性。在将新鲜和 6 个月冷冻血液与 FC 和 MC 进行比较时,我们观察到对任何细胞群的高度相关性和低偏差。这种相关性通过层次聚类得到证实。跨研究时间点的低变异系数 (CV) 表明样品保存良好长达 6 个月。细胞因子检测稳定性通过低 CV 得到证实,新鲜和固定条件之间存在一些差异。PROT 样品的 13 个月定期随访显示出显着的样品稳定性。如果仔细选择兼容的抗体组,可以保存全血用于表型分析和细胞因子反应研究。然而,细胞形态的可能变化、抗体亲和力的差异、应考虑与新鲜血液相比细胞因子阳性细胞频率的变化。我们的环境构成了多中心和回顾性研究的宝贵工具。© 2020 国际细胞计量学促进会


























 京公网安备 11010802027423号
京公网安备 11010802027423号