当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification two key residues at the intersection of domains of a thioether monooxygenase for improving its sulfoxidation performance
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-10-19 , DOI: 10.1002/bit.27604 Shi-Miao Ren 1 , Feng Liu 1 , Yin-Qi Wu 1 , Qi Chen 1 , Zhi-Jun Zhang 1 , Hui-Lei Yu 1 , Jian-He Xu 1
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-10-19 , DOI: 10.1002/bit.27604 Shi-Miao Ren 1 , Feng Liu 1 , Yin-Qi Wu 1 , Qi Chen 1 , Zhi-Jun Zhang 1 , Hui-Lei Yu 1 , Jian-He Xu 1
Affiliation
|
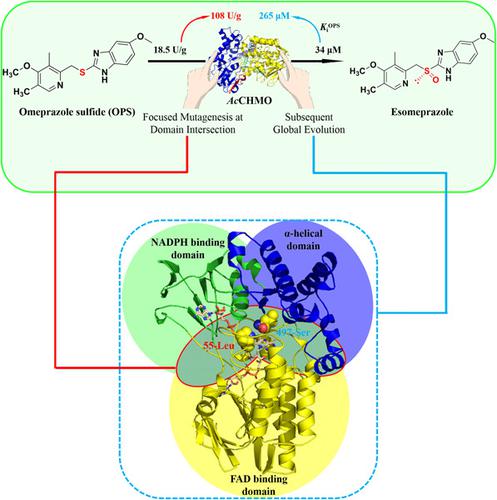
AcCHMO, a cyclohexanone monooxygenase from Acinetobacter calcoaceticus, is a typical Type I Baeyer–Villiger monooxygenase (BVMO). We previously obtained the AcCHMOM6 mutant, which oxidizes omeprazole sulfide (OPS) to the chiral sulfoxide drug esomeprazole. To further improve the catalytic efficiency of the AcCHMOM6 mutant, a focused mutagenesis strategy was adopted at the intersections of the FAD‐binding domain, NADPH‐binding domain, and α‐helical domain based on structural characteristics of AcCHMO. By using focused mutagenesis and subsequent global evolution two key residues (L55 and P497) at the intersections of the domains were identified. Mutant of L55Y improved catalytic efficiency significantly, whereas the P497S mutant alleviated substrate inhibition remarkably. AcCHMOM7 (L55Y/P497S) was obtained by combining the two mutations, which increased the specific activity from 18.5 (M6) to 108 U/g, and an increase in the Ki of the substrate OPS from 34 to 265 μM. The results indicate that catalytic performance can be elevated by modification of the sensitive sites at the intersection of the domains of AcCHMO. The results also provided some insights for the engineering of other Type I BVMOs or other multidomain proteins.
中文翻译:

鉴定硫醚单加氧酶结构域交叉处的两个关键残基以提高其亚砜化性能
Ac CHMO是一种来自醋酸钙不动杆菌的环己酮单加氧酶,是典型的 I 型 Baeyer-Villiger 单加氧酶 (BVMO)。我们之前获得了Ac CHMO M6突变体,该突变体将奥美拉唑硫化物 (OPS) 氧化为手性亚砜药物埃索美拉唑。为了进一步提高的催化效率AC CHMO M6突变体,诱变集中策略的FAD结合结构域的交叉点获得通过,NADPH结合域,和α螺旋结构域基于的结构特征ACCHMO。通过使用集中诱变和随后的全局进化,鉴定了域交叉处的两个关键残基(L55 和 P497)。L55Y 突变体显着提高了催化效率,而 P497S 突变体显着减轻了底物抑制。Ac CHMO M7 (L55Y/P497S) 是通过结合这两个突变获得的,其比活性从 18.5 (M6) 增加到 108 U/g,底物 OPS的K i从 34 增加到 265 μM。结果表明,催化性能可以通过修饰Ac域交叉处的敏感位点来提高。CHMO。结果还为其他 I 型 BVMO 或其他多域蛋白的工程设计提供了一些见解。
更新日期:2020-10-19
中文翻译:

鉴定硫醚单加氧酶结构域交叉处的两个关键残基以提高其亚砜化性能
Ac CHMO是一种来自醋酸钙不动杆菌的环己酮单加氧酶,是典型的 I 型 Baeyer-Villiger 单加氧酶 (BVMO)。我们之前获得了Ac CHMO M6突变体,该突变体将奥美拉唑硫化物 (OPS) 氧化为手性亚砜药物埃索美拉唑。为了进一步提高的催化效率AC CHMO M6突变体,诱变集中策略的FAD结合结构域的交叉点获得通过,NADPH结合域,和α螺旋结构域基于的结构特征ACCHMO。通过使用集中诱变和随后的全局进化,鉴定了域交叉处的两个关键残基(L55 和 P497)。L55Y 突变体显着提高了催化效率,而 P497S 突变体显着减轻了底物抑制。Ac CHMO M7 (L55Y/P497S) 是通过结合这两个突变获得的,其比活性从 18.5 (M6) 增加到 108 U/g,底物 OPS的K i从 34 增加到 265 μM。结果表明,催化性能可以通过修饰Ac域交叉处的敏感位点来提高。CHMO。结果还为其他 I 型 BVMO 或其他多域蛋白的工程设计提供了一些见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号