Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2020-10-19 , DOI: 10.1016/j.jece.2020.104621 Jianming Liu , Haohui Cui , Jianhua Li , Meichen Chen
|
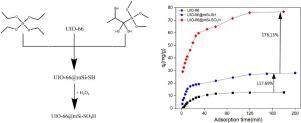
In this study, adsorption behavior on UIO-66, UIO-66@mSi-SH and UIO-66@mSi-SO3H were investigated with a great emphasis on cadmium. The UIO-66@mSi-SH was synthesized by postsynthetic modification with silica layer, and sulfo-functionalized UIO-66 with silica layer (UIO-66@mSi-SO3H) was obtained from oxidizing UIO-66@mSi-SH with H2O2. All prepared adsorbents were characterized by FT-IR, SEM, XRD and BET. The BET value of UIO-66 can reach 1304.59 m2 g−1. To fit the adsorption behavior curve of Cd2+, the isothermal adsorption curve (Langmuir, Freundlich and D-R models) and kinetic model (Quasi first-order kinetic, Quasi second-order kinetic and Elovich model) were utilized. It shows that the basic mechanism of adsorption process was determined by R2, AIC and other constants and the adsorption performance is shown as UIO-66@mSi-SO3H > UIO-66@mSi-SH > UIO-66. Langmuir and Elovich models showed better fitting results of UIO-66@mSi-SO3H adsorption process for Cd2+, and the R2 values were 0.97471 and 0.97817 respectively. The theoretical maximum adsorption capacity of UIO-66@mSi-SO3H for Cd2+ is 409.96 mg g-1 by Langmuir isothermal adsorption. The UIO-66@mSi-SO3H shows great adsorption capacity for Cd2+ with a surface area of 126.728 m2 g−1 and a pore volume of 0.102 cm3 g−1, which is approximately 93.73 % higher than UIO-66. Besides, more than 90 % adsorbents was able to be regenerated in shorter time after 5 cycles.
中文翻译:

水中二氧化硅层吸附巯基和磺基官能化的UIO-66的镉离子研究
在这项研究中,对UIO-66,UIO-66 @ mSi-SH和UIO-66 @ mSi-SO 3 H的吸附行为进行了研究,其中重点研究了镉。UIO-66 @ mSi-SH是通过对二氧化硅层进行后合成改性而合成的,然后通过用UIO-66 @ mSi-SH氧化UIO-66 @ mSi-SH得到磺化官能化的具有二氧化硅层的UIO-66(UIO-66 @ mSi-SO 3 H)。 H 2 O 2。通过FT-IR,SEM,XRD和BET对所有制备的吸附剂进行表征。UIO-66的BET值可以达到1304.59 m 2 g -1。拟合Cd 2+的吸附行为曲线,利用等温吸附曲线(Langmuir,Freundlich和DR模型)和动力学模型(拟一阶动力学,拟二阶动力学和Elovich模型)。结果表明,吸附过程的基本机理是由R 2,AIC等常数决定的,吸附性能表现为UIO-66 @ mSi-SO 3 H> UIO-66 @ mSi-SH> UIO-66。Langmuir和Elovich模型显示UIO-66 @ mSi-SO 3 H吸附Cd 2+的拟合效果更好,R 2值分别为0.97471和0.97817。UIO-66 @ mSi-SO 3 H对Cd 2+的理论最大吸附容量为409.96 mg g -1经Langmuir等温吸附。UIO-66 @ mSi-SO 3 H对Cd 2+的吸附能力强,表面积为126.728 m 2 g -1,孔体积为0.102 cm 3 g -1,比UIO-66高约93.73%。 66。此外,经过5个循环后,可以在更短的时间内再生出90%以上的吸附剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号