Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2020-10-19 , DOI: 10.1007/s10593-020-02797-z Ekaterina V. Sirotkina , Mariia M. Efremova , Galina L. Starova , Mikhail A. Kuznetsov , Alexander P. Molchanov
|
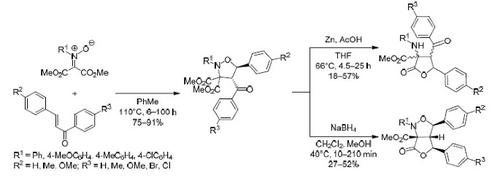
Ketonitrones containing two ester groups react regio- and stereoselectively with 1,3-diarylpropenones to form isoxazolidines with ester groups at position 3 of the ring. The action of zinc in acetic acid on these isoxazolidines causes opening of the ring with the formation of 3-amino alcohols, the subsequent cyclization of which leads to polysubstituted lactones. Reduction of the benzoyl group of isoxazolidine with sodium borohydride leads, as a result of subsequent transformations, to the formation of substituted 1,3,4-triaryl-6-oxodihydro-1H,3H-furo[3,4-c]isoxazole-6a(6H)-carboxylates as single diastereomers.
中文翻译:

将硝酮环加成为1,3-二芳基丙烯酮并随后转化生成的异恶唑烷
含有两个酯基的酮硝基酮与1,3-二芳基丙烯酮进行区域选择性和立体选择性反应,形成在环的3位带有酯基的异恶唑烷。乙酸中锌对这些异恶唑烷的作用导致开环,形成3-氨基醇,其后环化形成多取代的内酯。硼氢化钠还原异恶唑烷的苯甲酰基,由于随后的转化,导致形成取代的1,3,4-三芳基-6-恶二氢-1 H,3 H-呋喃[3,4- c ]异恶唑-6a(6 H)-羧酸盐为单一非对映异构体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号