当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unbiased proteomic screening identifies a novel role for the E3 ubiquitin ligase Nedd4-2 in translational suppression during ER stress
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2020-10-16 , DOI: 10.1111/jnc.15219 Daphne E Eagleman 1 , Jiuhe Zhu 1 , Dai-Chi Liu 2 , Joseph Seimetz 3 , Auinash Kalsotra 3, 4 , Nien-Pei Tsai 1, 2
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2020-10-16 , DOI: 10.1111/jnc.15219 Daphne E Eagleman 1 , Jiuhe Zhu 1 , Dai-Chi Liu 2 , Joseph Seimetz 3 , Auinash Kalsotra 3, 4 , Nien-Pei Tsai 1, 2
Affiliation
|
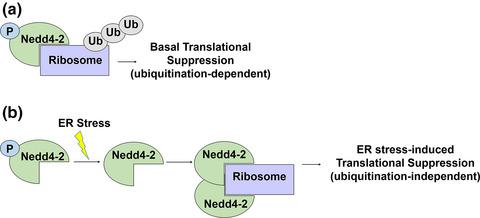
Endoplasmic reticulum (ER) stress occurs when protein folding or maturation is disrupted. A malfunction in the ER stress response can lead to cell death and has been observed in many neurological diseases. However, how the ER stress response is regulated in neuronal cells remains largely unclear. Here, we studied an E3 ubiquitin ligase named neural precursor cell expressed developmentally down-regulated protein 4-like (Nedd4-2). Nedd4-2 is highly expressed in the brain and has a high affinity toward ubiquitinating membrane-bound proteins. We first utilized unbiased proteomic profiling with ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) of isolated membrane fractions from mouse whole brains to identify novel targets of Nedd4-2. Through this screen, we found that the expression and ubiquitination of ribosomal proteins are regulated by Nedd4-2 and we confirmed an association between Nedd4-2 and ribosomes through ribosome sedimentation and polysome profiling. Further, we utilized immunoprecipitation and western blotting to show that induction of ER stress promotes an association between Nedd4-2 and ribosomal proteins, which is mediated through dephosphorylation of Nedd4-2 at serine-342. This increased interaction between Nedd4-2 and ribosomal proteins in turn mediates ER stress-associated translational suppression. In summary, the results of this study demonstrate a novel regulatory mechanism underlying the ER stress response and a novel function of Nedd4-2 in translational control. Our findings may shed light on neurological diseases in which the ER stress response or the function of Nedd4-2 is dysregulated.
中文翻译:

无偏见的蛋白质组学筛选确定了 E3 泛素连接酶 Nedd4-2 在 ER 应激期间的翻译抑制中的新作用
当蛋白质折叠或成熟被破坏时,会发生内质网 (ER) 应激。ER 应激反应中的故障可导致细胞死亡,并且已在许多神经系统疾病中观察到。然而,如何调节神经元细胞中的 ER 应激反应仍不清楚。在这里,我们研究了一种名为神经前体细胞的 E3 泛素连接酶,表达发育下调的蛋白 4 样 (Nedd4-2)。Nedd4-2 在大脑中高度表达,并且对泛素化膜结合蛋白具有高亲和力。我们首先利用来自小鼠全脑的分离膜组分的超高效液相色谱-串联质谱 (UPLC-MS/MS) 的无偏蛋白质组学分析来识别 Nedd4-2 的新靶点。通过这个画面,我们发现核糖体蛋白的表达和泛素化受 Nedd4-2 调节,我们通过核糖体沉降和多核糖体分析证实了 Nedd4-2 和核糖体之间的关联。此外,我们利用免疫沉淀和蛋白质印迹来表明 ER 应激的诱导促进了 Nedd4-2 和核糖体蛋白之间的关联,这是通过 Nedd4-2 在丝氨酸 342 处的去磷酸化介导的。Nedd4-2 和核糖体蛋白之间增加的相互作用反过来介导 ER 应激相关的翻译抑制。总之,本研究的结果证明了 ER 应激反应的新调节机制和 Nedd4-2 在翻译控制中的新功能。
更新日期:2020-10-16
中文翻译:

无偏见的蛋白质组学筛选确定了 E3 泛素连接酶 Nedd4-2 在 ER 应激期间的翻译抑制中的新作用
当蛋白质折叠或成熟被破坏时,会发生内质网 (ER) 应激。ER 应激反应中的故障可导致细胞死亡,并且已在许多神经系统疾病中观察到。然而,如何调节神经元细胞中的 ER 应激反应仍不清楚。在这里,我们研究了一种名为神经前体细胞的 E3 泛素连接酶,表达发育下调的蛋白 4 样 (Nedd4-2)。Nedd4-2 在大脑中高度表达,并且对泛素化膜结合蛋白具有高亲和力。我们首先利用来自小鼠全脑的分离膜组分的超高效液相色谱-串联质谱 (UPLC-MS/MS) 的无偏蛋白质组学分析来识别 Nedd4-2 的新靶点。通过这个画面,我们发现核糖体蛋白的表达和泛素化受 Nedd4-2 调节,我们通过核糖体沉降和多核糖体分析证实了 Nedd4-2 和核糖体之间的关联。此外,我们利用免疫沉淀和蛋白质印迹来表明 ER 应激的诱导促进了 Nedd4-2 和核糖体蛋白之间的关联,这是通过 Nedd4-2 在丝氨酸 342 处的去磷酸化介导的。Nedd4-2 和核糖体蛋白之间增加的相互作用反过来介导 ER 应激相关的翻译抑制。总之,本研究的结果证明了 ER 应激反应的新调节机制和 Nedd4-2 在翻译控制中的新功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号