当前位置:
X-MOL 学术
›
Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The SSV‐Seq 2.0 PCR‐Free Method Improves the Sequencing of Adeno‐Associated Viral Vector Genomes Containing GC‐Rich Regions and Homopolymers
Biotechnology Journal ( IF 4.7 ) Pub Date : 2020-10-16 , DOI: 10.1002/biot.202000016 Emilie Lecomte 1 , Sylvie Saleun 1 , Mathieu Bolteau 1 , Aurélien Guy‐Duché 1 , Oumeya Adjali 1 , Véronique Blouin 1 , Magalie Penaud‐Budloo 1 , Eduard Ayuso 1
Biotechnology Journal ( IF 4.7 ) Pub Date : 2020-10-16 , DOI: 10.1002/biot.202000016 Emilie Lecomte 1 , Sylvie Saleun 1 , Mathieu Bolteau 1 , Aurélien Guy‐Duché 1 , Oumeya Adjali 1 , Véronique Blouin 1 , Magalie Penaud‐Budloo 1 , Eduard Ayuso 1
Affiliation
|
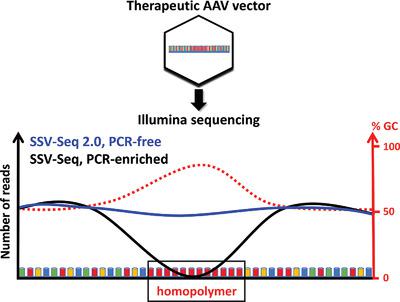
Adeno‐associated viral vectors (AAV) are efficient engineered tools for delivering genetic material into host cells. The commercialization of AAV‐based drugs must be accompanied by the development of appropriate quality control (QC) assays. Given the potential risk of co‐transfer of oncogenic or immunogenic sequences with therapeutic vectors, accurate methods to assess the level of residual DNA in AAV vector stocks are particularly important. An assay based on high‐throughput sequencing (HTS) to identify and quantify DNA species in recombinant AAV batches is developed. Here, it is shown that PCR amplification of regions that have a local GC content >90% and include successive mononucleotide stretches, such as the CAG promoter, can introduce bias during DNA library preparation, leading to drops in sequencing coverage. To circumvent this problem, SSV‐Seq 2.0, a PCR‐free protocol for sequencing AAV vector genomes containing such sequences, is developed. The PCR‐free protocol improves the evenness of the rAAV genome coverage and consequently leads to a more accurate relative quantification of residual DNA. HTS‐based assays provide a more comprehensive assessment of DNA impurities and AAV vector genome integrity than conventional QC tests based on real‐time PCR and are useful methods to improve the safety and efficacy of these viral vectors.
中文翻译:

SSV-Seq 2.0无PCR方法可改善包含GC丰富区域和均聚物的腺相关病毒载体基因组的测序
腺相关病毒载体(AAV)是将遗传物质传递到宿主细胞中的有效工程工具。基于AAV的药物的商业化必须伴随着适当的质量控制(QC)分析方法的发展。鉴于致癌或免疫原性序列与治疗性载体共转移的潜在风险,评估AAV载体原种中残留DNA水平的准确方法尤为重要。开发了一种基于高通量测序(HTS)的测定方法,以鉴定和定量重组AAV批次中的DNA种类。在此,显示了局部GC含量> 90%并包括连续的单核苷酸序列(如CAG启动子)的区域的PCR扩增可在DNA文库制备过程中引入偏倚,从而导致测序覆盖率下降。为了解决这个问题,开发了SSV-Seq 2.0,一种无需PCR的协议,可对包含此类序列的AAV载体基因组进行测序。无需PCR的方案可提高rAAV基因组覆盖范围的均匀性,从而使残留DNA的相对定量更加准确。与传统的基于实时PCR的QC检测相比,基于HTS的检测可对DNA杂质和AAV载体基因组完整性进行更全面的评估,是提高这些病毒载体的安全性和有效性的有用方法。
更新日期:2020-10-16
中文翻译:

SSV-Seq 2.0无PCR方法可改善包含GC丰富区域和均聚物的腺相关病毒载体基因组的测序
腺相关病毒载体(AAV)是将遗传物质传递到宿主细胞中的有效工程工具。基于AAV的药物的商业化必须伴随着适当的质量控制(QC)分析方法的发展。鉴于致癌或免疫原性序列与治疗性载体共转移的潜在风险,评估AAV载体原种中残留DNA水平的准确方法尤为重要。开发了一种基于高通量测序(HTS)的测定方法,以鉴定和定量重组AAV批次中的DNA种类。在此,显示了局部GC含量> 90%并包括连续的单核苷酸序列(如CAG启动子)的区域的PCR扩增可在DNA文库制备过程中引入偏倚,从而导致测序覆盖率下降。为了解决这个问题,开发了SSV-Seq 2.0,一种无需PCR的协议,可对包含此类序列的AAV载体基因组进行测序。无需PCR的方案可提高rAAV基因组覆盖范围的均匀性,从而使残留DNA的相对定量更加准确。与传统的基于实时PCR的QC检测相比,基于HTS的检测可对DNA杂质和AAV载体基因组完整性进行更全面的评估,是提高这些病毒载体的安全性和有效性的有用方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号