当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N‐pyridinylbenzamides: an isosteric approach towards new antimycobacterial compounds
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-10-17 , DOI: 10.1111/cbdd.13804 Daria Nawrot 1 , Eliška Suchánková 1 , Ondřej Janďourek 1 , Klára Konečná 1 , Pavel Bárta 1 , Martin Doležal 1 , Jan Zitko 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-10-17 , DOI: 10.1111/cbdd.13804 Daria Nawrot 1 , Eliška Suchánková 1 , Ondřej Janďourek 1 , Klára Konečná 1 , Pavel Bárta 1 , Martin Doležal 1 , Jan Zitko 1
Affiliation
|
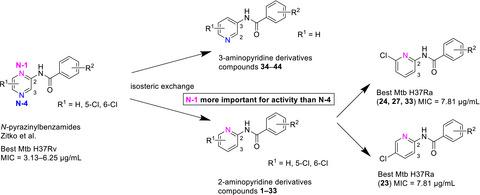
A series of N‐pyridinylbenzamides was designed and prepared to investigate the influence of isosterism and positional isomerism on antimycobacterial activity. Comparison to previously published isosteric N‐pyrazinylbenzamides was made as an attempt to draw structure–activity relationships in such type of compounds. In total, we prepared 44 different compounds, out of which fourteen had minimum inhibitory concentration (MIC) values against Mycobacterium tuberculosis H37Ra below 31.25 µg/ml, most promising being N‐(5‐chloropyridin‐2‐yl)‐3‐(trifluoromethyl)benzamide (23) and N‐(6‐chloropyridin‐2‐yl)‐3‐(trifluoromethyl)benzamide (24) with MIC = 7.81 µg/ml (26 µm). Five compounds showed broad‐spectrum antimycobacterial activity against M. tuberculosis H37Ra, M. smegmatis and M. aurum. N‐(pyridin‐2‐yl)benzamides were generally more active than N‐(pyridin‐3‐yl)benzamides, indicating that N‐1 in the parental structure of N‐pyrazinylbenzamides might be more important for antimycobacterial activity than N‐4. Marginal antibacterial and antifungal activity was observed for title compounds. The hepatotoxicity of title compounds was assessed in vitro on hepatocellular carcinoma cell line HepG2, and they may be considered non‐toxic (22 compounds with IC50 over 200 µm).
中文翻译:

N-吡啶基苯甲酰胺:一种新的抗分枝杆菌化合物的等排方法
设计并制备了一系列N-吡啶基苯甲酰胺,以研究等排和位置异构对抗分枝杆菌活性的影响。与先前发表的等排N-吡嗪基苯甲酰胺进行比较,试图绘制此类化合物的结构-活性关系。我们总共制备了 44 种不同的化合物,其中 14 种对结核分枝杆菌H37Ra 的最低抑菌浓度 (MIC) 值低于 31.25 µg/ml,最有希望的是N- (5-氯吡啶-2-基)-3-(三氟甲基)苯甲酰胺 ( 23 ) 和N- (6-氯吡啶-2-基)-3-(三氟甲基)苯甲酰胺 ( 24 ),MIC = 7.81 µg/ml (26 µg)米)。五种化合物对结核分枝杆菌H37Ra、耻垢分枝杆菌和金黄色分枝杆菌具有广谱抗分枝杆菌活性。N- (吡啶-2-基)苯甲酰胺通常比N- (吡啶-3-基)苯甲酰胺更具活性,表明N-吡嗪基苯甲酰胺亲本结构中的N -1可能比N-4对抗分枝杆菌活性更重要. 观察到标题化合物的边际抗菌和抗真菌活性。标题化合物的肝毒性在体外评估对肝癌细胞系HepG2,并且它们可以被认为是无毒的(22种化合物与IC 50超过200μ米)。
更新日期:2020-10-17
中文翻译:

N-吡啶基苯甲酰胺:一种新的抗分枝杆菌化合物的等排方法
设计并制备了一系列N-吡啶基苯甲酰胺,以研究等排和位置异构对抗分枝杆菌活性的影响。与先前发表的等排N-吡嗪基苯甲酰胺进行比较,试图绘制此类化合物的结构-活性关系。我们总共制备了 44 种不同的化合物,其中 14 种对结核分枝杆菌H37Ra 的最低抑菌浓度 (MIC) 值低于 31.25 µg/ml,最有希望的是N- (5-氯吡啶-2-基)-3-(三氟甲基)苯甲酰胺 ( 23 ) 和N- (6-氯吡啶-2-基)-3-(三氟甲基)苯甲酰胺 ( 24 ),MIC = 7.81 µg/ml (26 µg)米)。五种化合物对结核分枝杆菌H37Ra、耻垢分枝杆菌和金黄色分枝杆菌具有广谱抗分枝杆菌活性。N- (吡啶-2-基)苯甲酰胺通常比N- (吡啶-3-基)苯甲酰胺更具活性,表明N-吡嗪基苯甲酰胺亲本结构中的N -1可能比N-4对抗分枝杆菌活性更重要. 观察到标题化合物的边际抗菌和抗真菌活性。标题化合物的肝毒性在体外评估对肝癌细胞系HepG2,并且它们可以被认为是无毒的(22种化合物与IC 50超过200μ米)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号