当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fenton-like degradation enhancement of methylene blue dye with magnetic heating induction
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jelechem.2020.114773 F.L. Rivera , F.J. Recio , F.J. Palomares , J. Sánchez-Marcos , N. Menéndez , E. Mazarío , P. Herrasti
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jelechem.2020.114773 F.L. Rivera , F.J. Recio , F.J. Palomares , J. Sánchez-Marcos , N. Menéndez , E. Mazarío , P. Herrasti
|
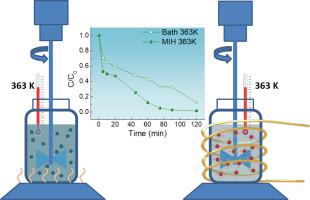
Abstract We studied the adsorption/degradation process of methylene blue in a Fenton like process using iron oxide nanoparticles as a source of Fe2+ ions, where the nanoparticles were prepared via a facile electrochemical synthesis method. The degradation kinetics were studied using 2 g L−1 of catalyst and 100 ppm of pollutant at pH 3.5. The influence of temperature on this process was evaluated using two different setups: conventional heating in a thermostatic bath and selective heating using an alternating magnetic field. The magnetic induction heating process led to a greater degradation of the pollutant compared with the thermostatic bath. In addition, the optimal concentration of Fe2+ in solution was evaluated in a Fenton homogeneous process to achieve the same degradation efficiency when using a nanoparticle-assisted Fenton-like process. A concentration of 0.5 ppm Fe2+ in solution yielded the same degradation achieved by using 2 g L−1 of iron oxide nanoparticles. The kinetic analysis fit the pseudo-first-order kinetics and indicated a linear increase in the apparent rate constant with increasing temperature. The activation energy of the degradation process obtained by fitting the Arrhenius equation was 58 kJ mol−1.
中文翻译:

磁热感应对亚甲蓝染料的类芬顿降解增强
摘要 我们研究了使用氧化铁纳米颗粒作为 Fe2+ 离子源的类芬顿工艺中亚甲蓝的吸附/降解过程,其中纳米颗粒是通过简便的电化学合成方法制备的。在 pH 3.5 下使用 2 g L-1 催化剂和 100 ppm 污染物研究降解动力学。使用两种不同的设置来评估温度对这个过程的影响:在恒温槽中的常规加热和使用交变磁场的选择性加热。与恒温浴相比,磁感应加热过程导致污染物的降解更大。此外,在 Fenton 均质过程中评估了溶液中 Fe2+ 的最佳浓度,以在使用纳米颗粒辅助的类 Fenton 过程时实现相同的降解效率。溶液中 0.5 ppm Fe2+ 的浓度产生了与使用 2 g L-1 的氧化铁纳米颗粒所实现的相同的降解。动力学分析符合准一级动力学,表明表观速率常数随温度升高呈线性增加。通过拟合阿伦尼乌斯方程得到的降解过程的活化能为 58 kJ mol-1。
更新日期:2020-12-01
中文翻译:

磁热感应对亚甲蓝染料的类芬顿降解增强
摘要 我们研究了使用氧化铁纳米颗粒作为 Fe2+ 离子源的类芬顿工艺中亚甲蓝的吸附/降解过程,其中纳米颗粒是通过简便的电化学合成方法制备的。在 pH 3.5 下使用 2 g L-1 催化剂和 100 ppm 污染物研究降解动力学。使用两种不同的设置来评估温度对这个过程的影响:在恒温槽中的常规加热和使用交变磁场的选择性加热。与恒温浴相比,磁感应加热过程导致污染物的降解更大。此外,在 Fenton 均质过程中评估了溶液中 Fe2+ 的最佳浓度,以在使用纳米颗粒辅助的类 Fenton 过程时实现相同的降解效率。溶液中 0.5 ppm Fe2+ 的浓度产生了与使用 2 g L-1 的氧化铁纳米颗粒所实现的相同的降解。动力学分析符合准一级动力学,表明表观速率常数随温度升高呈线性增加。通过拟合阿伦尼乌斯方程得到的降解过程的活化能为 58 kJ mol-1。


























 京公网安备 11010802027423号
京公网安备 11010802027423号