当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A copper-catalyzed asymmetric oxime propargylation enables the synthesis of the gliovirin tetrahydro-1,2-oxazine core
Chemical Science ( IF 8.4 ) Pub Date : 2020-10-15 , DOI: 10.1039/d0sc04802j Nicholas G W Cowper 1 , Matthew J Hesse 1 , Katie M Chan 1 , Sarah E Reisman 1
Chemical Science ( IF 8.4 ) Pub Date : 2020-10-15 , DOI: 10.1039/d0sc04802j Nicholas G W Cowper 1 , Matthew J Hesse 1 , Katie M Chan 1 , Sarah E Reisman 1
Affiliation
|
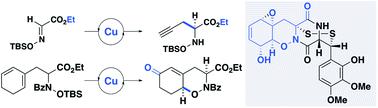
The bicyclic tetrahydro-1,2-oxazine subunit of gliovirin is synthesized through a diastereoselective copper-catalyzed cyclization of an N-hydroxyamino ester. Oxidative elaboration to the fully functionalized bicycle was achieved through a series of mild transformations. Central to this approach was the development of the first catalytic, enantioselective propargylation of an oxime to furnish a key N-hydroyxamino ester intermediate.
中文翻译:

铜催化的不对称肟炔丙基化能够合成格里奥韦林四氢-1,2-恶嗪核心
格里奥韦林的双环四氢-1,2-恶嗪亚基是通过N-羟基氨基酯的非对映选择性铜催化环化合成的。通过一系列温和的转变,氧化精制而成了功能齐全的自行车。该方法的核心是开发了肟的第一个催化对映选择性炔丙基化反应,以提供关键的N-羟基氨基酯中间体。
更新日期:2020-10-15
中文翻译:

铜催化的不对称肟炔丙基化能够合成格里奥韦林四氢-1,2-恶嗪核心
格里奥韦林的双环四氢-1,2-恶嗪亚基是通过N-羟基氨基酯的非对映选择性铜催化环化合成的。通过一系列温和的转变,氧化精制而成了功能齐全的自行车。该方法的核心是开发了肟的第一个催化对映选择性炔丙基化反应,以提供关键的N-羟基氨基酯中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号