European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-10-15 , DOI: 10.1016/j.ejmech.2020.112935 Zelin Yang , Xin Huang , Wenfang Lai , Yuheng Tang , Junjie Liu , Yingzheng Wang , Kedan Chu , John Brown , Guizhu Hong
|
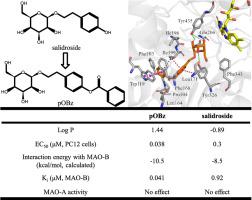
Salidroside [(2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-(4-hydroxyphenethoxy)tetrahy-dro-2H-pyran-3,4,5-triol] is an antioxidant, anti-inflammatory and neuroprotective agent, but its drug-like properties are unoptimized and its mechanism of actions is uncertain. We synthesized twenty-six novel derivatives of salidroside and examined them in CoCl2-treated PC12 cells using MTT assay. pOBz, synthesized by esterifying the phenolic hydroxyl group of salidroside with benzoyl chloride, was one of five derivatives that were more cytoprotective than salidroside, with an EC50 of 0.038 μM versus 0.30 μM for salidroside. pOBz was also more lipophilic, with log P of 1.44 versus −0.89 for salidroside. Reverse virtual docking predicted that pOBz would bind strongly with monoamine oxidase (MAO) B by occupying its entrance and substrate cavities, and by interacting with the inter-cavity gating residue Ile199 and Tyr435 of the substrate cavity. Enzymatic studies confirmed that pOBz competitively inhibited the activity of purified human MAO-B (Ki = 0.041 μM versus Ki = 0.92 μM for salidroside), and pOBz was highly selective for MAO-B over MAO-A. In vivo, pOBz inhibited cerebral MAO activity after middle cerebral artery occlusion with reperfusion in rats, and it reduced cerebral infarct volume, improved neurological function and NeuN expression, and inhibited complement C3 expression and apoptosis. Our results suggest that pOBz is a structurally novel type of competitive and selective MAO-B inhibitor, with potent neuroprotective properties after cerebral ischemia-reperfusion injury in rats.
中文翻译:

红景天苷新衍生物的合成和鉴定,该衍生物为具有增强的神经保护特性的单胺氧化酶B的选择性竞争抑制剂
红景天苷[(2R,3S,4S,5R,6R)-2-(羟甲基)-6-(4-羟基苯乙氧基)四氢-dro-2H-吡喃-3,4,5-三醇]是抗氧化剂,抗炎药和神经保护剂,但其类药物性质尚未优化,其作用机理尚不确定。我们合成了二十六个新的红景天苷衍生物,并使用MTT法在CoCl 2处理的PC12细胞中对其进行了检查。通过将红景天苷的酚羟基与苯甲酰氯酯化而合成的pOBz是五种比红景天苷具有更强细胞保护作用的衍生物之一,其EC 500.038μM,而红景天苷为0.30μM。pOBz也更具亲脂性,log P为1.44,而红景天苷为-0.89。反向虚拟对接预测,pOBz通过占据其入口腔和底物腔,并与腔间门控残基Ile199和Tyr435相互作用,与单胺氧化酶(MAO)B牢固结合。酶的研究证实,pOBz竞争性抑制纯化的人MAO-B的活性(K我 = 0.041μM与ķ我 = 0.92μM为红景天)和pOBz是为MAO-B,经MAO-A具有高度选择性。体内,pOBz抑制大鼠中脑动脉再灌注后脑MAO活性,并减少脑梗塞体积,改善神经功能和NeuN表达,并抑制补体C3表达和细胞凋亡。我们的结果表明,pOBz是一种结构新颖的竞争性和选择性MAO-B抑制剂,在大鼠脑缺血-再灌注损伤后具有有效的神经保护作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号