当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cation-exchange mediated synthesis of hydrogen and sodium titanates heterojunction: Theoretical and experimental insights toward photocatalyic mechanism
Applied Surface Science ( IF 6.7 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148137 Isabela M. Iani , Vinícius Teodoro , Naiara L. Marana , Ubirajara Coleto , Julio R. Sambrano , Alexandre Z. Simões , Marcio D. Teodoro , Elson Longo , Leinig A. Perazolli , Rafael A. C. Amoresi , Maria Aparecida Zaghete
Applied Surface Science ( IF 6.7 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148137 Isabela M. Iani , Vinícius Teodoro , Naiara L. Marana , Ubirajara Coleto , Julio R. Sambrano , Alexandre Z. Simões , Marcio D. Teodoro , Elson Longo , Leinig A. Perazolli , Rafael A. C. Amoresi , Maria Aparecida Zaghete
|
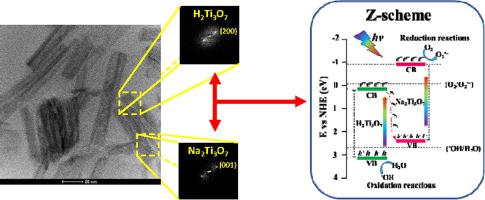
Abstract Na2Ti3O7/H2Ti3O7 heterojunction nanotubes were obtained through the microwave-assisted hydrothermal method (MAH) with different band gap energy engineering. This study aimed to synthesize sodium titanate nanotubes and evaluate the influence of H+ insertion on their photocatalytic properties. Heterojunctions were identified by X-ray diffraction, Raman spectroscopy and high-resolution transmission electron microscopy, and their electronic levels and defects were investigated using diffuse reflectance and photoluminescence spectroscopies in the UV-Vis region. The cation exchange process promotes the formation of Na2Ti3O7/H2Ti3O7 heterojunction with coexistence of both phases in the nanotube. Photocatalytic results of Rhodamine-B (Rh-B) dye discoloration show that the prepared materials have activity under visible and UV light irradiation, and are dependent on the proportion of hydrogen and sodium-titanate phases present. The material with highest sodium concentration showed discoloration with a half-life time of 23 minutes under visible light irradiation. Theoretical results reveal the heterojunction band offset as a staggered gap, with the effective bandgap occurring between the O-2p of Na2Ti3O7 and Ti-3d of H2Ti3O7. The charge carrier transfer mechanism in the heterojunctions is well described by Z-scheme, with H2Ti3O7 and Na2Ti3O7 as the main oxidation and reduction phases for dye discoloration.
中文翻译:

阳离子交换介导的氢和钛酸钠异质结合成:光催化机理的理论和实验见解
摘要 通过不同带隙能量工程的微波辅助水热法(MAH)获得了Na2Ti3O7/H2Ti3O7异质结纳米管。本研究旨在合成钛酸钠纳米管并评估 H+ 插入对其光催化性能的影响。通过 X 射线衍射、拉曼光谱和高分辨率透射电子显微镜识别异质结,并在紫外-可见光区域使用漫反射和光致发光光谱研究它们的电子能级和缺陷。阳离子交换过程促进了 Na2Ti3O7/H2Ti3O7 异质结的形成,同时纳米管中的两相共存。罗丹明-B(Rh-B)染料变色的光催化结果表明,制备的材料在可见光和紫外光照射下具有活性,并且取决于存在的氢和钛酸钠相的比例。钠浓度最高的材料在可见光照射下变色,半衰期为 23 分钟。理论结果显示异质结带偏移为交错间隙,有效带隙发生在 Na2Ti3O7 的 O-2p 和 H2Ti3O7 的 Ti-3d 之间。Z-scheme 很好地描述了异质结中的电荷载流子转移机制,其中 H2Ti3O7 和 Na2Ti3O7 作为染料变色的主要氧化和还原相。理论结果显示异质结带偏移为交错间隙,有效带隙发生在 Na2Ti3O7 的 O-2p 和 H2Ti3O7 的 Ti-3d 之间。Z-scheme 很好地描述了异质结中的电荷载流子转移机制,其中 H2Ti3O7 和 Na2Ti3O7 作为染料变色的主要氧化和还原相。理论结果显示异质结带偏移为交错间隙,有效带隙发生在 Na2Ti3O7 的 O-2p 和 H2Ti3O7 的 Ti-3d 之间。Z-scheme 很好地描述了异质结中的电荷载流子转移机制,其中 H2Ti3O7 和 Na2Ti3O7 作为染料变色的主要氧化和还原相。
更新日期:2021-02-01
中文翻译:

阳离子交换介导的氢和钛酸钠异质结合成:光催化机理的理论和实验见解
摘要 通过不同带隙能量工程的微波辅助水热法(MAH)获得了Na2Ti3O7/H2Ti3O7异质结纳米管。本研究旨在合成钛酸钠纳米管并评估 H+ 插入对其光催化性能的影响。通过 X 射线衍射、拉曼光谱和高分辨率透射电子显微镜识别异质结,并在紫外-可见光区域使用漫反射和光致发光光谱研究它们的电子能级和缺陷。阳离子交换过程促进了 Na2Ti3O7/H2Ti3O7 异质结的形成,同时纳米管中的两相共存。罗丹明-B(Rh-B)染料变色的光催化结果表明,制备的材料在可见光和紫外光照射下具有活性,并且取决于存在的氢和钛酸钠相的比例。钠浓度最高的材料在可见光照射下变色,半衰期为 23 分钟。理论结果显示异质结带偏移为交错间隙,有效带隙发生在 Na2Ti3O7 的 O-2p 和 H2Ti3O7 的 Ti-3d 之间。Z-scheme 很好地描述了异质结中的电荷载流子转移机制,其中 H2Ti3O7 和 Na2Ti3O7 作为染料变色的主要氧化和还原相。理论结果显示异质结带偏移为交错间隙,有效带隙发生在 Na2Ti3O7 的 O-2p 和 H2Ti3O7 的 Ti-3d 之间。Z-scheme 很好地描述了异质结中的电荷载流子转移机制,其中 H2Ti3O7 和 Na2Ti3O7 作为染料变色的主要氧化和还原相。理论结果显示异质结带偏移为交错间隙,有效带隙发生在 Na2Ti3O7 的 O-2p 和 H2Ti3O7 的 Ti-3d 之间。Z-scheme 很好地描述了异质结中的电荷载流子转移机制,其中 H2Ti3O7 和 Na2Ti3O7 作为染料变色的主要氧化和还原相。


























 京公网安备 11010802027423号
京公网安备 11010802027423号