当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Designed Streptococcus pyogenes Sortase A Accepts Branched Amines as Nucleophiles in Sortagging
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-10-14 , DOI: 10.1021/acs.bioconjchem.0c00486 Zhi Zou 1, 2 , Maximilian Nöth 1, 2 , Felix Jakob 1, 2 , Ulrich Schwaneberg 1, 2
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-10-14 , DOI: 10.1021/acs.bioconjchem.0c00486 Zhi Zou 1, 2 , Maximilian Nöth 1, 2 , Felix Jakob 1, 2 , Ulrich Schwaneberg 1, 2
Affiliation
|
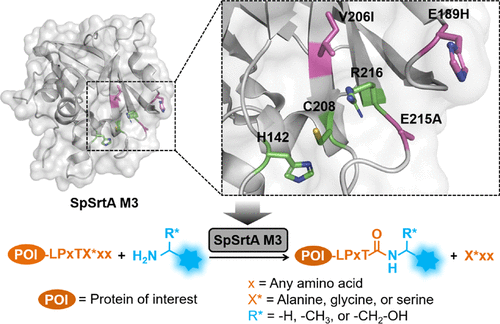
Sortase-mediated ligation (sortagging) is commonly performed using the Staphylococcus aureus sortase A (SaSrtA) that strictly recognizes the N-terminal glycine residue. In this work, a rational design of Streptococcus pyogenes sortase A (SpSrtA) for improved transpeptidase activity toward different N-terminal amino acid residues was conducted. The generated variant SpSrtA M3 (E189H/V206I/E215A) showed up to 6.6-fold (vs SpSrtA wild-type) enhanced catalytic efficiency. Additionally, M3 retains the specificity toward N-terminal alanine, glycine, serine residues, as well as branched (at α-carbon) primary amines as wild-type parent. Furthermore, M3 was applied for head-to-tail backbone cyclization of proteins.
中文翻译:

设计的化脓性链球菌分选酶A在分选中接受支链胺作为亲核试剂
分选酶介导的连接(分选)通常使用严格识别N末端甘氨酸残基的金黄色葡萄球菌分选酶A(SaSrtA)进行。在这项工作中,进行了化脓性链球菌分选酶A(SpSrtA)的合理设计,以提高针对不同N末端氨基酸残基的转肽酶活性。生成的变体SpSrtA M3(E189H / V206I / E215A)显示高达6.6倍的催化效率(相对于SpSrtA野生型)。另外,M3保留了对N末端丙氨酸,甘氨酸,丝氨酸残基以及支链(在α-碳原子上)伯胺作为野生型亲本的特异性。此外,M3被用于蛋白质从头到尾的主链环化。
更新日期:2020-11-18
中文翻译:

设计的化脓性链球菌分选酶A在分选中接受支链胺作为亲核试剂
分选酶介导的连接(分选)通常使用严格识别N末端甘氨酸残基的金黄色葡萄球菌分选酶A(SaSrtA)进行。在这项工作中,进行了化脓性链球菌分选酶A(SpSrtA)的合理设计,以提高针对不同N末端氨基酸残基的转肽酶活性。生成的变体SpSrtA M3(E189H / V206I / E215A)显示高达6.6倍的催化效率(相对于SpSrtA野生型)。另外,M3保留了对N末端丙氨酸,甘氨酸,丝氨酸残基以及支链(在α-碳原子上)伯胺作为野生型亲本的特异性。此外,M3被用于蛋白质从头到尾的主链环化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号