当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvent‐free liquid avidin as a step toward cold chain elimination
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-10-14 , DOI: 10.1002/bit.27587 Liem Bui-Le 1 , Alex P S Brogan 2 , Jason P Hallett 1
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-10-14 , DOI: 10.1002/bit.27587 Liem Bui-Le 1 , Alex P S Brogan 2 , Jason P Hallett 1
Affiliation
|
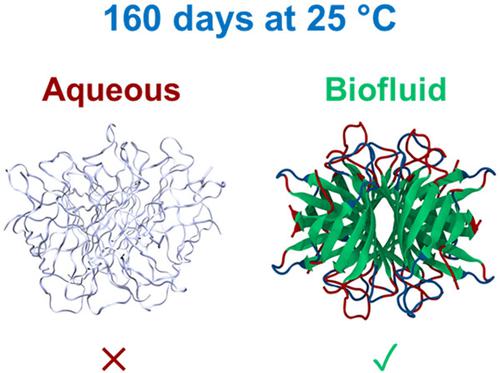
The temperature sensitivity of vaccines and therapeutic proteins forces the distribution of life‐saving treatments to rely heavily on the temperature‐controlled (usually 2–8°C) supply and distribution network known as the cold chain. Here, using avidin as a model, we demonstrate how surface engineering could significantly increase the thermal stability of therapeutic proteins. A combination of spectroscopic (Fourier transform infrared, circular dichroism, and ultraviolet‐visible) and scattering techniques (dynamic light scattering, small‐angle, and wide‐angle X‐ray scattering) were deployed to probe the activity, structure, and stability of the model protein. Temperature‐dependent synchrotron radiation circular dichroism spectroscopy was used to demonstrate a significant increase in thermal stability, with a half denaturation temperature of 139.0°C and reversible unfolding with modified avidin returning to a 90% folded state when heated to temperatures below 100°C. Accelerated aging studies revealed that modified avidin retained its secondary structure after storage at 40°C for 56 days, equivalent to 160 days at 25°C. Furthermore, binding studies with multiple ligands revealed that the binding site remained functional after modification. As a result, this approach has potential as a storage technology for therapeutic proteins and the elimination of the cold chain, enabling the dissemination of life‐saving vaccines worldwide.
中文翻译:

无溶剂液体亲和素作为消除冷链的一步
疫苗和治疗性蛋白质的温度敏感性迫使挽救生命的治疗方法的分配严重依赖于称为冷链的温度控制(通常为 2-8°C)的供应和分配网络。在这里,使用抗生物素蛋白作为模型,我们展示了表面工程如何显着提高治疗性蛋白质的热稳定性。结合光谱(傅里叶变换红外、圆二色性和紫外-可见光)和散射技术(动态光散射、小角和广角 X 射线散射)来探测活性、结构和稳定性。模型蛋白。温度相关的同步辐射圆二色光谱用于证明热稳定性显着增加,半变性温度为 139。0°C 和可逆展开,当加热到 100°C 以下的温度时,修饰的亲和素恢复到 90% 的折叠状态。加速老化研究表明,经过修饰的亲和素在 40°C 下储存 56 天后仍保留其二级结构,相当于在 25°C 下储存 160 天。此外,与多个配体的结合研究表明,结合位点在修饰后仍保持功能。因此,这种方法具有作为治疗性蛋白质的储存技术和消除冷链的潜力,从而能够在全球范围内传播拯救生命的疫苗。相当于 25°C 下 160 天。此外,与多个配体的结合研究表明,结合位点在修饰后仍保持功能。因此,这种方法具有作为治疗性蛋白质的储存技术和消除冷链的潜力,从而能够在全球范围内传播拯救生命的疫苗。相当于 25°C 下 160 天。此外,与多个配体的结合研究表明,结合位点在修饰后仍保持功能。因此,这种方法具有作为治疗性蛋白质的储存技术和消除冷链的潜力,从而能够在全球范围内传播拯救生命的疫苗。
更新日期:2020-10-14
中文翻译:

无溶剂液体亲和素作为消除冷链的一步
疫苗和治疗性蛋白质的温度敏感性迫使挽救生命的治疗方法的分配严重依赖于称为冷链的温度控制(通常为 2-8°C)的供应和分配网络。在这里,使用抗生物素蛋白作为模型,我们展示了表面工程如何显着提高治疗性蛋白质的热稳定性。结合光谱(傅里叶变换红外、圆二色性和紫外-可见光)和散射技术(动态光散射、小角和广角 X 射线散射)来探测活性、结构和稳定性。模型蛋白。温度相关的同步辐射圆二色光谱用于证明热稳定性显着增加,半变性温度为 139。0°C 和可逆展开,当加热到 100°C 以下的温度时,修饰的亲和素恢复到 90% 的折叠状态。加速老化研究表明,经过修饰的亲和素在 40°C 下储存 56 天后仍保留其二级结构,相当于在 25°C 下储存 160 天。此外,与多个配体的结合研究表明,结合位点在修饰后仍保持功能。因此,这种方法具有作为治疗性蛋白质的储存技术和消除冷链的潜力,从而能够在全球范围内传播拯救生命的疫苗。相当于 25°C 下 160 天。此外,与多个配体的结合研究表明,结合位点在修饰后仍保持功能。因此,这种方法具有作为治疗性蛋白质的储存技术和消除冷链的潜力,从而能够在全球范围内传播拯救生命的疫苗。相当于 25°C 下 160 天。此外,与多个配体的结合研究表明,结合位点在修饰后仍保持功能。因此,这种方法具有作为治疗性蛋白质的储存技术和消除冷链的潜力,从而能够在全球范围内传播拯救生命的疫苗。


























 京公网安备 11010802027423号
京公网安备 11010802027423号