当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One‐Step Synthesis of Acylboron Compounds via Copper‐Catalyzed Carbonylative Borylation of Alkyl Halides**
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-10-14 , DOI: 10.1002/anie.202012373 Li‐Jie Cheng 1 , Siling Zhao 1 , Neal P. Mankad 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-10-14 , DOI: 10.1002/anie.202012373 Li‐Jie Cheng 1 , Siling Zhao 1 , Neal P. Mankad 1
Affiliation
|
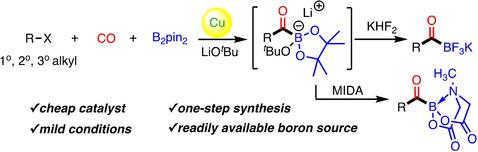
A copper‐catalyzed carbonylative borylation of unactivated alkyl halides has been developed, enabling efficient synthesis of aliphatic potassium acyltrifluoroborates (KATs) in high yields by treating the in situ formed tetracoordinated acylboron intermediates with aqueous KHF2. A variety of functional groups are tolerated under the mild reaction conditions, and primary, secondary, and tertiary alkyl halides are all applicable. In addition, this method also provides facile access to N‐methyliminodiacetyl (MIDA) acylboronates as well as α‐methylated potassium acyltrifluoroborates in a one‐pot manner. Mechanistic studies indicate a radical atom transfer carbonylation (ATC) mechanism to form acyl halide intermediates that are subsequently borylated by (NHC)CuBpin.
中文翻译:

通过铜催化烷基卤化羰基化硼化一步合成酰基硼化合物**
已开发出未活化烷基卤的铜催化羰基化硼酸酯化反应,可通过用KHF 2水溶液处理原位形成的四配位酰基中间体来高效合成脂肪族酰基三氟硼酸钾(KAT)。在温和的反应条件下可耐受多种官能团,伯,仲和叔烷基卤化物均适用。此外,该方法还可以单锅方式轻松获得N-甲基亚氨基二乙酰(MIDA)酰基硼酸酯和α-甲基化酰基三氟硼酸钾。机理研究表明,自由基原子转移羰基化(ATC)机理形成酰基卤中间体,随后被(NHC)CuBpin硼化。
更新日期:2020-10-14
中文翻译:

通过铜催化烷基卤化羰基化硼化一步合成酰基硼化合物**
已开发出未活化烷基卤的铜催化羰基化硼酸酯化反应,可通过用KHF 2水溶液处理原位形成的四配位酰基中间体来高效合成脂肪族酰基三氟硼酸钾(KAT)。在温和的反应条件下可耐受多种官能团,伯,仲和叔烷基卤化物均适用。此外,该方法还可以单锅方式轻松获得N-甲基亚氨基二乙酰(MIDA)酰基硼酸酯和α-甲基化酰基三氟硼酸钾。机理研究表明,自由基原子转移羰基化(ATC)机理形成酰基卤中间体,随后被(NHC)CuBpin硼化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号