当前位置:
X-MOL 学术
›
Int. J. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An ESI Q-TOF study to understand the impact of arginine on CID MS/MS characteristics of polypeptides
International Journal of Mass Spectrometry ( IF 1.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.ijms.2020.116453 Pandi Boomathi Pandeswari , Varatharajan Sabareesh
International Journal of Mass Spectrometry ( IF 1.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.ijms.2020.116453 Pandi Boomathi Pandeswari , Varatharajan Sabareesh
|
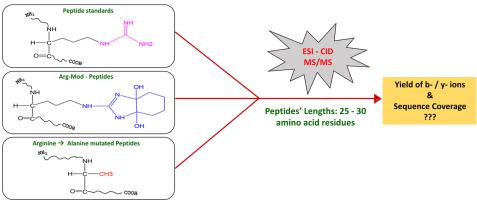
Abstract To understand the importance of arginine (Arg) in influencing electrospray ionization (ESI) - collision induced dissociation (CID) tandem mass spectrometry (MS/MS) behavior of peptides of lengths > ∼ 25 amino acid residues (a.a.r), we chose carbamidomethylated Insulin B-chain (30 a.a.r), glucagon (29 a.a.r) and melittin (26 a.a.r) as the models for this investigation. Also, two smaller peptides: Angiotensin II (8 a.a.r) and Bradykinin (9 a.a.r) were studied for better comprehension of the interplay between the influence of Arg and peptide’s length. The motivation to study such longer peptides stems from middle-down proteomics, a recently emerging field encompassing investigations on proteolytic peptides longer than ∼25 a.a.r. CID MS/MS data of two different cases have been compared: (1) standard model peptides vs. chemically modified peptides, wherein sidechain guanidine group of Arg residues in these model peptides are selectively modified by 1,2-cyclohexanedione and phenylglyoxal; (2) standard model peptides vs. mutated model peptides, in which Arg residues in these model peptides are substituted by alanine (Ala) residues (Arg → Ala). Different types of stoichiometric products were obtained due to this chemical modification and each type of arginine-modified product was subjected to ESI CID MS/MS. The Arg → Ala mutated model peptides chosen for this study are: [R22A]-Insulin B-chain, [R17A & R18A]-Glucagon, [R22A & R24A]-Melittin and a shorter peptide: R2A-Angiotensin II. All experiments were performed in a quadrupole time-of-flight hybrid mass spectrometer, whereby CID (hexapole collision cell) was done by following fixed collision energy (CE) as well as ramped CE. Analysis of CID MS/MS spectra revealed that the sequence coverage of Arg → Ala mutated peptides was higher than the chemically modified peptides as well as their respective standard (unmodified) peptides. Examination of all the MS/MS data alluded that Arg has a greater influence on the CID MS/MS behavior of longer peptides, viz., lengths > 25 a.a.r, than the smaller peptides. .
中文翻译:

一项 ESI Q-TOF 研究,以了解精氨酸对多肽 CID MS/MS 特征的影响
摘要 为了了解精氨酸 (Arg) 在影响长度 > ∼ 25 个氨基酸残基 (aar) 的肽的电喷雾电离 (ESI) - 碰撞诱导解离 (CID) 串联质谱 (MS/MS) 行为中的重要性,我们选择了氨基甲酰甲基化胰岛素 B 链 (30 aar)、胰高血糖素 (29 aar) 和蜂毒肽 (26 aar) 作为该研究的模型。此外,还研究了两种较小的肽:血管紧张素 II (8 aar) 和缓激肽 (9 aar),以更好地理解 Arg 影响与肽长度之间的相互作用。研究这种更长肽的动机源于中下蛋白质组学,这是一个最近出现的领域,包括对两种不同情况下长度超过 25 aar CID MS/MS 数据的蛋白水解肽的研究:(1)标准模型肽与 化学修饰的肽,其中这些模型肽中Arg残基的侧链胍基被1,2-环己二酮和苯乙二醛选择性修饰;(2) 标准模型肽 vs. 突变模型肽,其中这些模型肽中的 Arg 残基被丙氨酸 (Ala) 残基取代 (Arg → Ala)。由于这种化学改性,获得了不同类型的化学计量产物,并且每种类型的精氨酸改性产物都进行了 ESI CID MS/MS。本研究选择的 Arg → Ala 突变模型肽是:[R22A]-胰岛素 B 链、[R17A & R18A]-胰高血糖素、[R22A & R24A]-蜂毒肽和较短的肽:R2A-血管紧张素 II。所有实验均在四极杆飞行时间混合质谱仪上进行,其中 CID(六极杆碰撞池)是通过遵循固定碰撞能量 (CE) 以及斜坡 CE 来完成的。CID MS/MS 谱分析表明,Arg → Ala 突变肽的序列覆盖率高于化学修饰肽及其各自的标准(未修饰)肽。对所有 MS/MS 数据的检查表明,与较小的肽相比,Arg 对较长肽(即长度 > 25 aar)的 CID MS/MS 行为的影响更大。. 25 aar,比较小的肽。. 25 aar,比较小的肽。.
更新日期:2021-01-01
中文翻译:

一项 ESI Q-TOF 研究,以了解精氨酸对多肽 CID MS/MS 特征的影响
摘要 为了了解精氨酸 (Arg) 在影响长度 > ∼ 25 个氨基酸残基 (aar) 的肽的电喷雾电离 (ESI) - 碰撞诱导解离 (CID) 串联质谱 (MS/MS) 行为中的重要性,我们选择了氨基甲酰甲基化胰岛素 B 链 (30 aar)、胰高血糖素 (29 aar) 和蜂毒肽 (26 aar) 作为该研究的模型。此外,还研究了两种较小的肽:血管紧张素 II (8 aar) 和缓激肽 (9 aar),以更好地理解 Arg 影响与肽长度之间的相互作用。研究这种更长肽的动机源于中下蛋白质组学,这是一个最近出现的领域,包括对两种不同情况下长度超过 25 aar CID MS/MS 数据的蛋白水解肽的研究:(1)标准模型肽与 化学修饰的肽,其中这些模型肽中Arg残基的侧链胍基被1,2-环己二酮和苯乙二醛选择性修饰;(2) 标准模型肽 vs. 突变模型肽,其中这些模型肽中的 Arg 残基被丙氨酸 (Ala) 残基取代 (Arg → Ala)。由于这种化学改性,获得了不同类型的化学计量产物,并且每种类型的精氨酸改性产物都进行了 ESI CID MS/MS。本研究选择的 Arg → Ala 突变模型肽是:[R22A]-胰岛素 B 链、[R17A & R18A]-胰高血糖素、[R22A & R24A]-蜂毒肽和较短的肽:R2A-血管紧张素 II。所有实验均在四极杆飞行时间混合质谱仪上进行,其中 CID(六极杆碰撞池)是通过遵循固定碰撞能量 (CE) 以及斜坡 CE 来完成的。CID MS/MS 谱分析表明,Arg → Ala 突变肽的序列覆盖率高于化学修饰肽及其各自的标准(未修饰)肽。对所有 MS/MS 数据的检查表明,与较小的肽相比,Arg 对较长肽(即长度 > 25 aar)的 CID MS/MS 行为的影响更大。. 25 aar,比较小的肽。. 25 aar,比较小的肽。.

























 京公网安备 11010802027423号
京公网安备 11010802027423号