Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2020-10-14 , DOI: 10.1016/j.xcrp.2020.100222 Qing-Hua Li , Dingding Gao , Cheng-Yu He , Qi Liao , Yun-Xuan Tan , Yu-Hui Wang , Rui Ding , Guo-Qiang Lin , Ping Tian
|
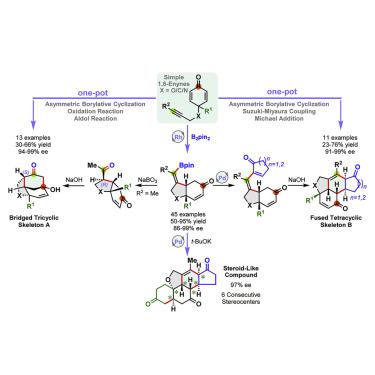
Bridged and fused polycyclic structures are found in a huge number of natural products. Significant progress has been made on their syntheses; however, facile and practical strategies to afford polycycles with tunable substituents and functional groups remain rare. Here, we report a practical rhodium(III)-catalyzed asymmetric borylative cyclization of cyclohexadienone-containing 1,6-enynes, affording highly enantioenriched cis-bicyclic frameworks with great functional group compatibility, and further extend it to construct bridged tricyclic and fused tetracyclic skeletons in one-pot protocols. The combination of borylative cyclization with a subsequent oxidation/aldol reaction sequence goes through an epimerization of the stereocenter adjacent to the acetyl group, yielding various tricyclic compounds. In addition, borylative cyclization is combined with a Suzuki-Miyaura coupling/Michael addition sequence, leading to diverse tetracyclic compounds. Starting from simple starting materials, both one-pot transformations yield complex products bearing several consecutive stereocenters with excellent enantioselectivities. Furthermore, an unnatural steroid-like compound is also readily prepared, demonstrating the potential of this methodology.
中文翻译:

通过铑(III)催化的不对称硼化环化反应一锅法制备桥接的三环和稠合四环支架
在大量天然产物中发现了桥连和稠合的多环结构。它们的合成已取得重大进展;然而,提供具有可调取代基和官能团的多环的简便可行的策略仍然很少。在这里,我们报告实用的铑(III)催化的含环己二酮的1,6-炔烃的不对称硼化环化,提供高度对映体富集的顺式-双环框架具有强大的官能团兼容性,并进一步扩展以在一锅协议中构建桥接的三环和稠合四环骨架。硼化环化与随后的氧化/羟醛反应序列的组合经历了邻近乙酰基的立体中心的差向异构化,产生了各种三环化合物。另外,硼烷基化与铃木-宫浦偶合/迈克尔加成序列相结合,产生各种四环化合物。从简单的起始原料开始,两种一锅转化都可产生带有多个连续立体中心且具有出色对映选择性的复杂产物。此外,还可以容易地制备出非天然类固醇样化合物,证明了这种方法的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号