当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of Human 15-Lipoxygenase-2 in the Biosynthesis of the Lipoxin Intermediate, 5S,15S-diHpETE, Implicated with the Altered Positional Specificity of Human 15-Lipoxygenase-1
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-13 , DOI: 10.1021/acs.biochem.0c00622 Steven C Perry 1 , Thomas Horn 1 , Benjamin E Tourdot 2 , Adriana Yamaguchi 2 , Chakrapani Kalyanaraman 3 , William S Conrad 1 , Oluwayomi Akinkugbe 1 , Michael Holinstat 2 , Matthew P Jacobson 3 , Theodore R Holman 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-13 , DOI: 10.1021/acs.biochem.0c00622 Steven C Perry 1 , Thomas Horn 1 , Benjamin E Tourdot 2 , Adriana Yamaguchi 2 , Chakrapani Kalyanaraman 3 , William S Conrad 1 , Oluwayomi Akinkugbe 1 , Michael Holinstat 2 , Matthew P Jacobson 3 , Theodore R Holman 1
Affiliation
|
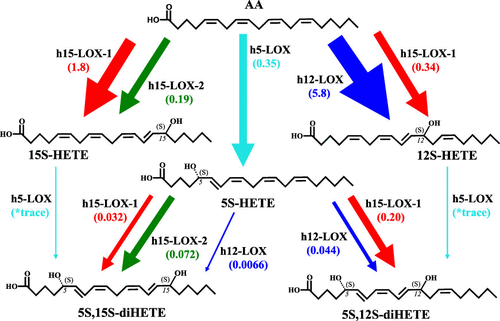
The oxylipins, 5S,12S-dihydroxy-6E,8Z,10E,14Z-eicosatetraenoic acid (5S,12S-diHETE) and 5S,15S-dihydroxy-6E,8Z,11Z,13E-eicosatetraenoic acid (5S,15S-diHETE), have been identified in cell exudates and have chemotactic activity toward eosinophils and neutrophils. Their biosynthesis has been proposed to occur by sequential oxidations of arachidonic acid (AA) by lipoxygenase enzymes, specifically through oxidation of AA by h5-LOX followed by h12-LOX, h15-LOX-1, or h15-LOX-2. In this work, h15-LOX-1 demonstrates altered positional specificity when reacting with 5S-HETE, producing 90% 5S,12S-diHETE, instead of 5S,15S-diHETE, with kinetics 5-fold greater than that of h12-LOX. This is consistent with previous work in which h15-LOX-1 reacts with 7S-HDHA, producing the noncanonical, DHA-derived, specialized pro-resolving mediator, 7S,14S-diHDHA. It is also determined that oxygenation of 5S-HETE by h15-LOX-2 produces 5S,15S-diHETE and its biosynthetic kcat/KM flux is 2-fold greater than that of h15-LOX-1, suggesting that h15-LOX-2 may have a greater role in lipoxin biosynthesis than previously thought. In addition, it is shown that oxygenation of 12S-HETE and 15S-HETE by h5-LOX is kinetically slow, suggesting that the first step in the in vitro biosynthesis of both 5S,12S-diHETE and 5S,15S-diHETE is the production of 5S-HETE.
中文翻译:

人 15-Lipoxygenase-2 在脂氧素中间体 5S,15S-diHpETE 生物合成中的作用,与人 15-Lipoxygenase-1 的位置特异性改变有关
oxylipins, 5S,12S-dihydroxy-6E,8Z,10E,14Z-eicosatetraenoic acid (5S,12S-diHETE) 和 5S,15S-dihydroxy-6E,8Z,11Z,13E-eicosatetraenoic acid (5S,15S-diHETE) , 已在细胞渗出液中得到鉴定,并且对嗜酸性粒细胞和中性粒细胞具有趋化活性。已经提出它们的生物合成是通过脂肪氧化酶对花生四烯酸 (AA) 的顺序氧化而发生的,特别是通过 h5-LOX 氧化 AA,然后是 h12-LOX、h15-LOX-1 或 h15-LOX-2。在这项工作中,h15-LOX-1 在与 5S-HETE 反应时表现出改变的位置特异性,产生 90% 5S,12S-diHETE,而不是 5S,15S-diHETE,其动力学是 h12-LOX 的 5 倍。这与之前的工作一致,其中 h15-LOX-1 与 7S-HDHA 反应,产生非规范的、DHA 衍生的、专门的促分辨介质 7S,14S-diHDHA。k cat / K M通量是 h15-LOX-1 的 2 倍,表明 h15-LOX-2 在脂氧素生物合成中的作用可能比以前认为的更大。此外,研究表明 h5-LOX 对 12S-HETE 和 15S-HETE 的氧化在动力学上是缓慢的,这表明5S,12S-diHETE 和 5S,15S-diHETE体外生物合成的第一步是产生5S-HETE。
更新日期:2020-10-28
中文翻译:

人 15-Lipoxygenase-2 在脂氧素中间体 5S,15S-diHpETE 生物合成中的作用,与人 15-Lipoxygenase-1 的位置特异性改变有关
oxylipins, 5S,12S-dihydroxy-6E,8Z,10E,14Z-eicosatetraenoic acid (5S,12S-diHETE) 和 5S,15S-dihydroxy-6E,8Z,11Z,13E-eicosatetraenoic acid (5S,15S-diHETE) , 已在细胞渗出液中得到鉴定,并且对嗜酸性粒细胞和中性粒细胞具有趋化活性。已经提出它们的生物合成是通过脂肪氧化酶对花生四烯酸 (AA) 的顺序氧化而发生的,特别是通过 h5-LOX 氧化 AA,然后是 h12-LOX、h15-LOX-1 或 h15-LOX-2。在这项工作中,h15-LOX-1 在与 5S-HETE 反应时表现出改变的位置特异性,产生 90% 5S,12S-diHETE,而不是 5S,15S-diHETE,其动力学是 h12-LOX 的 5 倍。这与之前的工作一致,其中 h15-LOX-1 与 7S-HDHA 反应,产生非规范的、DHA 衍生的、专门的促分辨介质 7S,14S-diHDHA。k cat / K M通量是 h15-LOX-1 的 2 倍,表明 h15-LOX-2 在脂氧素生物合成中的作用可能比以前认为的更大。此外,研究表明 h5-LOX 对 12S-HETE 和 15S-HETE 的氧化在动力学上是缓慢的,这表明5S,12S-diHETE 和 5S,15S-diHETE体外生物合成的第一步是产生5S-HETE。



























 京公网安备 11010802027423号
京公网安备 11010802027423号