当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Optimized Scalable Synthesis of Chiral Iridium Pyridyl‐Phosphinite (Pyridophos) Catalysts
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-10-13 , DOI: 10.1002/hlca.202000181 Marc‐André Müller 1 , Adnan Ganić 1 , Esther Hörmann 1 , Stefan Kaiser 1 , Matthias Maywald 1 , Stephen J. Roseblade 1 , Marcus G. Schrems 1 , Andreas Schumacher 1 , David Woodmansee 1 , Andreas Pfaltz 1
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-10-13 , DOI: 10.1002/hlca.202000181 Marc‐André Müller 1 , Adnan Ganić 1 , Esther Hörmann 1 , Stefan Kaiser 1 , Matthias Maywald 1 , Stephen J. Roseblade 1 , Marcus G. Schrems 1 , Andreas Schumacher 1 , David Woodmansee 1 , Andreas Pfaltz 1
Affiliation
|
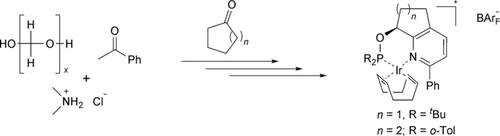
Iridium catalysts with chiral P,N ligands have greatly enhanced the scope of asymmetric olefin hydrogenation because they do not require a coordinating group near the C=C bond like Rh and Ru catalysts. Pyridophos ligands, possessing a conformationally restricted annulated pyridine framework linked to a phosphinite group, proved to be particularly effective, inducing high enantioselectivities in the hydrogenation of a remarkably broad range of substrates. Here we report the development of an efficient scalable synthesis for the two most versatile Ir‐pyridophos catalysts, derived from 2‐phenyl‐8‐hydroxy‐5,6,7,8‐tetrahydroquinoline or the analogue with a five‐membered carbocyclic ring, respectively, by modification and optimization of the original synthetic route. The optimized route renders both catalysts readily accessible in multi‐gram quantities in analytically pure form in overall yields of 26–37 %, starting from acetophenone and cyclopentanone or cyclohexanone, respectively. A major advantage of the new synthesis is the efficient and practical kinetic resolution of the late‐stage pyridyl alcohol intermediates with commercial immobilized Candida antarctica lipase B, giving access to both enantiomers of these catalysts as essentially enantiopure compounds. The catalysts are obtained as crystalline solids, which are air‐stable and can be stored for years at −20 °C without notable decomposition.
中文翻译:

优化的手性铱吡啶-磷鎓铱(Pyridophos)催化剂的可扩展合成
具有手性P,N配体的铱催化剂极大地增加了不对称烯烃氢化的范围,因为它们不需要像Rh和Ru催化剂那样在C = C键附近的配位基团。具有与次亚膦酸酯基团连接的构象受限的吡啶环骨架的吡咯磷配体被证明是特别有效的,在显着范围广泛的底物的氢化中诱导了高对映选择性。在这里,我们报告了一种高效可扩展的合成方法,该方法适用于两种最通用的Ir-吡啶甲酸催化剂,这些催化剂衍生自2-苯基-8-羟基-5,6,7,8-四氢喹啉或具有五元碳环的类似物,分别通过修改和优化原始合成路线。优化的路线使两种催化剂都可以容易地以分析纯的形式以克数形式获得,总产率为26-37%,分别从苯乙酮和环戊酮或环己酮开始。新合成法的主要优点是高效和实用的动力学拆分,可实现工业化固定化的后期吡啶醇中间体南极假丝酵母脂肪酶B,可以使这些催化剂的两种对映异构体基本为对映纯化合物。催化剂以结晶固体形式获得,它们是空气稳定的,可以在-20°C下保存多年,而不会发生明显的分解。
更新日期:2020-12-11
中文翻译:

优化的手性铱吡啶-磷鎓铱(Pyridophos)催化剂的可扩展合成
具有手性P,N配体的铱催化剂极大地增加了不对称烯烃氢化的范围,因为它们不需要像Rh和Ru催化剂那样在C = C键附近的配位基团。具有与次亚膦酸酯基团连接的构象受限的吡啶环骨架的吡咯磷配体被证明是特别有效的,在显着范围广泛的底物的氢化中诱导了高对映选择性。在这里,我们报告了一种高效可扩展的合成方法,该方法适用于两种最通用的Ir-吡啶甲酸催化剂,这些催化剂衍生自2-苯基-8-羟基-5,6,7,8-四氢喹啉或具有五元碳环的类似物,分别通过修改和优化原始合成路线。优化的路线使两种催化剂都可以容易地以分析纯的形式以克数形式获得,总产率为26-37%,分别从苯乙酮和环戊酮或环己酮开始。新合成法的主要优点是高效和实用的动力学拆分,可实现工业化固定化的后期吡啶醇中间体南极假丝酵母脂肪酶B,可以使这些催化剂的两种对映异构体基本为对映纯化合物。催化剂以结晶固体形式获得,它们是空气稳定的,可以在-20°C下保存多年,而不会发生明显的分解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号