当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics of D,L–Lactide Polymerization Initiated with Zirconium Acetylacetonate
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-10-13 , DOI: 10.1002/open.202000101 Kirill T Kalinin 1 , Nikita G Sedush 1 , Petr V Dmitryakov 1 , Sergei N Chvalun 1
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-10-13 , DOI: 10.1002/open.202000101 Kirill T Kalinin 1 , Nikita G Sedush 1 , Petr V Dmitryakov 1 , Sergei N Chvalun 1
Affiliation
|
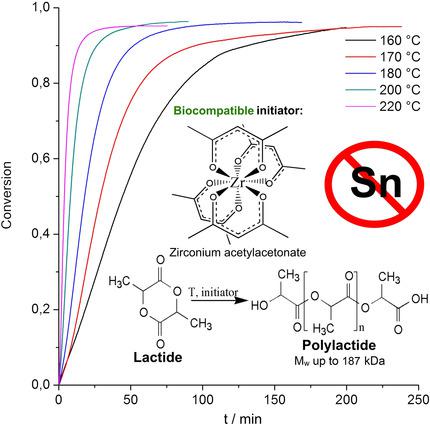
The kinetic of D,L‐lactide polymerization in presence of biocompatible zirconium acetylacetonate initiator was studied by differential scanning calorimetry in isothermal mode at various temperatures and initiator concentrations. The enthalpy of D,L‐lactide polymerization measured directly in DSC cell was found to be ΔH=−17.8±1.4 kJ mol−1. Kinetic curves of D,L‐lactide polymerization and propagation rate constants were determined for polymerization with zirconium acetylacetonate at concentrations of 250–1000 ppm and temperature of 160–220 °C. Using model or reversible polymerization the following kinetic and thermodynamic parameters were calculated: activation energy Ea=44.51±5.35 kJ mol−1, preexponential constant lnA=15.47±1.38, entropy of polymerization ΔS=−25.14 J mol−1 K−1. The effect of reaction conditions on the molecular weight of poly(D,L‐lactide) was shown.
中文翻译:

乙酰丙酮锆引发的 D,L-丙交酯聚合动力学
通过差示扫描量热法在等温模式下在不同温度和引发剂浓度下研究了生物相容性乙酰丙酮锆引发剂存在下 D,L-丙交酯聚合的动力学。直接在 DSC 池中测量的 D,L-丙交酯聚合的焓被发现为ΔH =−17.8±1.4 kJ mol −1。测定了浓度为 250-1000 ppm、温度为 160-220 °C 的乙酰丙酮锆聚合反应的 D,L-丙交酯聚合动力学曲线和增长速率常数。使用模型或可逆聚合计算以下动力学和热力学参数:活化能E a =44.51±5.35 kJ mol -1,指前常数ln A =15.47±1.38,聚合熵ΔS =−25.14 J mol -1 K -1。显示了反应条件对聚(D,L-丙交酯)分子量的影响。
更新日期:2020-10-13
中文翻译:

乙酰丙酮锆引发的 D,L-丙交酯聚合动力学
通过差示扫描量热法在等温模式下在不同温度和引发剂浓度下研究了生物相容性乙酰丙酮锆引发剂存在下 D,L-丙交酯聚合的动力学。直接在 DSC 池中测量的 D,L-丙交酯聚合的焓被发现为ΔH =−17.8±1.4 kJ mol −1。测定了浓度为 250-1000 ppm、温度为 160-220 °C 的乙酰丙酮锆聚合反应的 D,L-丙交酯聚合动力学曲线和增长速率常数。使用模型或可逆聚合计算以下动力学和热力学参数:活化能E a =44.51±5.35 kJ mol -1,指前常数ln A =15.47±1.38,聚合熵ΔS =−25.14 J mol -1 K -1。显示了反应条件对聚(D,L-丙交酯)分子量的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号