Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-10-13 , DOI: 10.1016/j.bbamem.2020.183488 Narek Yousefian , Alina Ornik-Cha , Sylvie Poussard , Marion Decossas , Melanie Berbon , Laetitia Daury , Jean-Christophe Taveau , Jean-William Dupuy , Selena Đorđević-Marquardt , Olivier Lambert , Klaas M. Pos
|
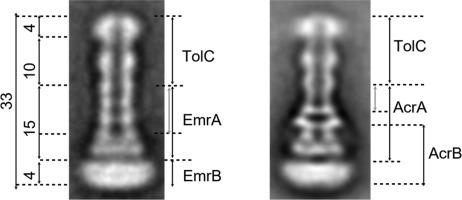
Gram-negative bacteria export a large variety of antimicrobial compounds by forming two-membrane spanning tripartite multidrug efflux systems composed of an inner membrane transporter, an outer membrane channel and a periplasmic adaptor protein. Here we present the co-expression, purification and first electron microscopy insights of the Escherichia coli EmrAB–TolC tripartite Major Facilitator Superfamily (MSF) efflux system as a whole complex stabilized by Amphipol polymer. The structure reveals a 33 nm long complex delineated by the Amphipol belt at both extremities. Comparison of projection structures of EmrAB-TolC and AcrAB-TolC indicates that the outer membrane protein TolC linked to the periplasmic adaptor EmrA protein form an extended periplasmic canal. The overall length of EmrAB-TolC complex is similar to that of AcrAB-TolC with a probable tip-to-tip interaction between EmrA and TolC unveiling how the adaptor protein connects TolC and EmrB embedded in the inner membrane.
中文翻译:

大肠杆菌EmrAB-TolC外排复合物的结构表征
革兰氏阴性细菌通过形成由内膜转运蛋白,外膜通道和周质衔接蛋白组成的跨越两方的跨三方多药外排系统,输出多种抗菌化合物。在这里,我们介绍了大肠杆菌的共表达,纯化和首次电子显微镜观察EmrAB–TolC三方主要促进剂超家族(MSF)外排体系是由Amphipol聚合物稳定的整体复合物。该结构揭示了在两肢由Amphipol带划定的33 nm长的复合物。EmrAB-TolC和AcrAB-TolC的投影结构比较表明,与周质适配器EmrA蛋白连接的外膜蛋白TolC形成了一条延伸的周质管。EmrAB-TolC复合体的总长度与AcrAB-TolC的总长度相似,EmrA和TolC之间可能存在尖端到尖端的相互作用,这揭示了衔接蛋白如何连接TolC和内膜中嵌入的EmrB。



























 京公网安备 11010802027423号
京公网安备 11010802027423号