Letters in Drug Design & Discovery ( IF 1 ) Pub Date : 2020-09-30 , DOI: 10.2174/1570180817999200513091613 Leyla Yurttaş 1 , Gülşen Akalin Çiftçi 2 , Mehmet Onur Aksoy 2 , Şeref Demirayak 3
|
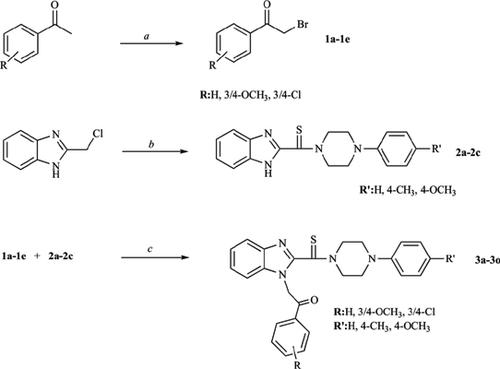
Background: Benzimidazole derivatives are privileged molecules known to have a wide variety of biological activities. In medicinal chemistry, due to the ring’s structural similarity to nucleotides, its derivatives were investigated as new chemotherapeutic agents. Our research group have been studying 1,2-disubstituted benzimidazoles, including thiocarbamoyl group and their potential anticancer activity. Based on previous findings, we synthesized novel 1-[2-(4-substituted phenyl-2-oxoethyl)]-2-[(2/3/4-substituted phenylpiperidin-1-yl)thiocarbamoyl]benzimidazole derivatives (3a-o).
Methods: The obtained fifteen derivatives were studied on A549 adenocarcinomic human alveolar basal epithelial cell line and mouse L929 fibroblastic cell line to determine their cytotoxic activity. These compounds were also investigated to identify their apoptotic properties.
Results and Discussion: The structures of the compounds based on three different groups differ from each other with the phenyl substituents bonded to the piperazine ring. All of the compounds showed remarkable antitumor activity, but the first five compounds bearing non-substituted phenyl moiety exhibited selective cytotoxicity when compared in terms of potencies to the normal cell line.
Conclusion: Compounds 3j, 3m and 3n were identified as the most apoptotic derivatives; however, compounds 3e and 3h provoked apoptosis with the percentages of 10.6 and 10.9% and selective cytotoxicity.
中文翻译:

新型苯并咪唑衍生物:肺癌细胞系的细胞毒性和凋亡特性
背景:苯并咪唑衍生物是特权分子,已知具有多种生物活性。在药物化学中,由于该环与核苷酸的结构相似,因此对其衍生物进行了研究,作为新的化学治疗剂。我们的研究小组一直在研究1,2-二取代的苯并咪唑类化合物,包括硫代氨基甲酰基及其潜在的抗癌活性。基于以前的发现,我们合成了新型的1- [2-(4-取代的苯基-2-氧代乙基)]-2-[((2/3 / 4-取代的苯基哌啶-1-基)硫代氨基甲酰基]苯并咪唑衍生物(3a-o )。
方法:在人肺腺癌A549腺泡基底上皮细胞系和小鼠L929成纤维细胞系上研究获得的十五种衍生物的细胞毒性。还研究了这些化合物以鉴定其凋亡特性。
结果与讨论:基于三个不同基团的化合物的结构彼此不同,苯基取代基键合到哌嗪环上。所有这些化合物均显示出显着的抗肿瘤活性,但是与常规细胞系相比,前五个带有未取代苯基部分的化合物表现出选择性的细胞毒性。
结论:化合物3j,3m和3n被确定为凋亡最强的衍生物。然而,化合物3e和3h引起细胞凋亡,其百分数为10.6和10.9%,并具有选择性细胞毒性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号